The landscape of chemistry is vast and intricate, populated with a myriad of terms and concepts that offer insights into the composition and behavior of substances at the molecular level. Among these, the terms “congener” and “isomer” stand out for their significance in understanding chemical diversity and specificity. These concepts not only provide a basis for categorizing chemical species but also play a crucial role in the fields of pharmaceuticals, environmental science, and even in the food and beverage industry.
Congeners are chemicals that are related by origin, possessing a similar chemical structure or belonging to the same chemical family, yet differing in composition to some degree. Isomers, on the other hand, refer to compounds with the same molecular formula but different arrangements of atoms within the molecule, leading to variations in their chemical properties. The distinction between these two concepts is fundamental in chemistry for the classification and study of organic compounds.
Delving into the specifics, congeners and isomers reveal the complexity and versatility of chemical compounds. Congeners often share biological activities or toxicological characteristics, making them particularly interesting in environmental chemistry and pharmacology. Isomers, with their structural and spatial diversity, highlight the intricacy of chemical bonds and the significance of molecular structure in determining the properties and functions of substances. Understanding these differences not only enriches our knowledge of chemistry but also has practical implications in drug development, environmental protection, and the creation of flavors and fragrances.

Basic Concepts
Chemical Structure
Definition and Significance
The chemical structure of a compound is the foundation of chemistry. It describes the arrangement of atoms in a molecule, including both the spatial orientation and the bonds between these atoms. Understanding a compound’s chemical structure is vital because it determines the properties and behaviors of the substance, such as its reactivity, polarity, and phase (solid, liquid, or gas under standard conditions).
Molecular Composition
Atoms and Molecules Overview
At the core of chemical structures are atoms, the basic units of matter, which combine in various ways to form molecules. Atoms consist of a nucleus, made up of protons and neutrons, surrounded by electrons. The way these electrons are shared or transferred between atoms forms chemical bonds, leading to the creation of molecules. The molecular composition not only tells us which atoms are present but also how many of each, providing a snapshot of the substance’s basic chemical identity.
Congeners Explained
Definition
Congeners are chemicals that belong to the same family, sharing a common structural framework but differing slightly in composition. This slight variation can significantly affect the chemical behavior and biological activity of each congener.
Basic Understanding of Congeners
Congeners arise from either natural processes or synthetic production. They often share basic chemical and physical properties but exhibit unique characteristics due to their differences in structure.
Examples
Common examples of congeners include:
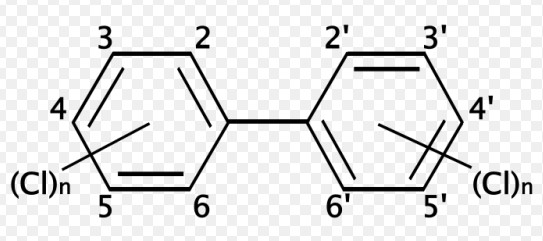
- Chlorinated biphenyls (PCBs), where each congener has a different number of chlorine atoms.
- Alcohols in beverages, where ethanol is the primary alcohol, but other congeners contribute to the taste and aroma.
Applications
Congeners have varied roles across several fields:
- Pharmaceuticals: Different congeners of a drug can have varying therapeutic effects and side effects.
- Beverages: The congeners in wine and spirits affect their flavor profiles.
- Environmental Science: Identifying specific congeners of pollutants helps in assessing environmental damage and health risks.
Isomers Detailed
Definition
Isomers are compounds with the same molecular formula but different arrangements of atoms. This difference in structure leads to distinct physical and chemical properties.
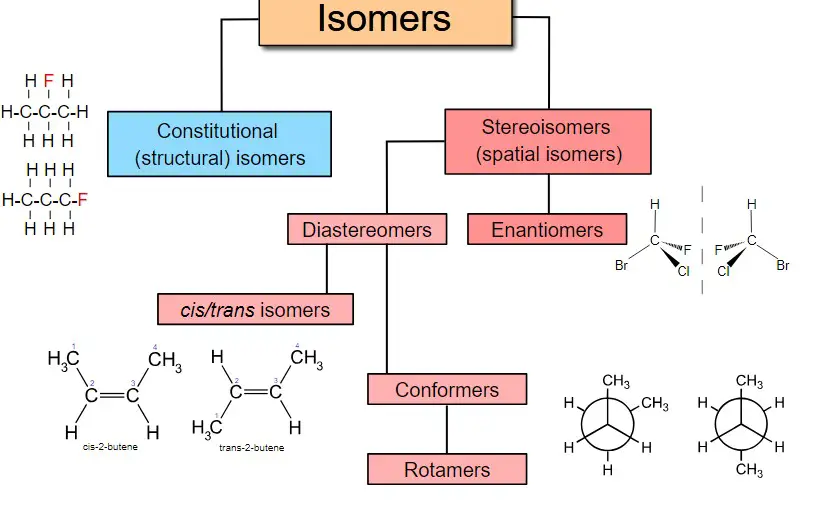
Types of Isomers
Isomers can be broadly categorized into structural isomers and stereoisomers.
Structural Isomers
Structural isomers have different connectivities among their atoms. They can be further classified based on the type of variation:
- Chain isomers: Different arrangements of the carbon backbone.
- Position isomers: Functional groups attached at different positions on the carbon chain.
- Functional group isomers: Different functional groups formed by rearranging the atoms.
Stereoisomers
Stereoisomers have the same connectivities but differ in the spatial orientation of atoms. The main types are:
- Geometric isomers: Differ in their arrangement around a double bond or ring structure.
- Optical isomers: Molecules that are mirror images of each other but are not superimposable.
Examples
Practical examples of isomers include:
- Butane and isobutane: Structural isomers where butane has a straight chain, and isobutane has a branched chain.
- Cis-trans isomers of alkenes: Geometric isomers where the substituents are on the same side (cis) or opposite sides (trans) of a double bond.
- Glucose and fructose: Functional group isomers where both have the formula C6H12O6, but glucose is an aldose, and fructose is a ketose.
Congeners vs. Isomers
Key Differences
When examining the distinctions between congeners and isomers, it is essential to first highlight their definitions. Congeners are compounds within the same family that have similar but not identical chemical structures. In contrast, isomers are compounds with the same molecular formula but different arrangements of atoms or orientation in space. This fundamental difference underscores the diversity of chemical compounds and their behaviors.
Comparison of Definitions
Congeners often share a core structural motif but vary in some aspects of their composition, leading to differences in properties and functions. Isomers, however, have an identical chemical formula but can differ drastically in both their physical and chemical properties due to the different arrangements of atoms or spatial orientations.
Molecular Structure
The molecular structure reveals more about the contrasts between congeners and isomers. For congeners, the variance often lies in the substitution on a common framework, affecting only a part of the molecule’s overall structure and function. Isomers demonstrate a broader range of structural diversity because their atoms can be connected in different ways or arranged differently in space, leading to entirely different molecules.
Practical Implications
The differences in applications and relevance of congeners and isomers are profound. Congeners play a crucial role in the nuances of flavor and aroma in food and beverages, as well as in the toxicity of environmental pollutants. Isomers, with their structural differences, are vital in pharmaceuticals where the efficacy and safety of drugs can depend on the specific isomer of a compound.
Significance in Science
Chemical Synthesis
In chemical synthesis, the distinction between congeners and isomers is crucial. Synthesizing specific congeners of a compound can target the creation of substances with desired properties, such as increased potency or reduced toxicity. For isomers, synthesis strategies must carefully control the arrangement of atoms to achieve the intended isomeric form, which can have dramatically different biological or chemical activity.
Environmental Impact
The understanding of pollutants and contaminants significantly benefits from distinguishing between congeners and isomers. Certain congeners of industrial chemicals, such as PCBs, have been identified as more harmful than others, informing regulatory actions and cleanup efforts. Similarly, the environmental fate of different isomers of a substance can vary, affecting how they are monitored and managed.
Pharmaceutical Relevance
In drug development and efficacy, the role of congeners and isomers is paramount. Pharmaceuticals often target specific congeners of a bioactive compound to fine-tune the drug’s effects. Similarly, the selection of a particular isomer can enhance a drug’s efficacy, reduce side effects, and influence its metabolism within the body, making isomerism a critical consideration in pharmaceutical design.
Industry Applications
Food and Beverage
In the food and beverage industry, congeners are responsible for the flavor profiles and aroma of products. The subtle differences between congeners can significantly affect the sensory experience of foods and drinks. Safety standards also consider the presence and concentration of specific congeners, especially in alcoholic beverages, due to their impact on health.
Flavor Profiles and Safety Standards
- Congeners in whiskey, for example, contribute to its complex flavor and aroma.
- Regulatory bodies monitor and regulate the levels of certain congeners to ensure product safety and quality.
Pharmaceuticals
In pharmaceuticals, both congeners and isomers have critical roles in drug design and therapeutic effects. The process involves selecting the right congener or isomer of a compound to achieve desired medicinal properties with minimal side effects.
Drug Design and Therapeutic Effects
- The choice between different isomers of a drug can influence its interaction with biological targets, absorption, distribution, metabolism, and excretion (ADME) properties.
- Developing drugs as a specific enantiomer (a type of stereoisomer) can improve efficacy and reduce toxicity, highlighting the importance of isomerism in modern pharmacology.
Frequently Asked Questions
What Are Congeners in Chemistry?
Congeners in chemistry are compounds within the same family that share a common structural feature, but differ in composition, properties, or both. These variations often result in different biological or chemical behaviors among congeners, making them significant in pharmaceuticals, where subtle differences can alter drug efficacy and safety.
How Do Isomers Differ from Each Other?
Isomers differ from each other in the arrangement of atoms within their molecules, despite having the same molecular formula. This difference in structure can lead to distinct properties, such as boiling points, melting points, and reactivity. Isomers are categorized into structural isomers, with different connectivity of atoms, and stereoisomers, which differ in spatial arrangement.
Why Are Congeners and Isomers Important?
Congeners and isomers are crucial in chemistry for understanding the diversity and specificity of chemical compounds. They aid in the development of drugs by allowing chemists to fine-tune molecular structures for desired effects, in environmental science to track pollution sources, and in the food and beverage industry to create or enhance flavors and aromas.
How Are Isomers Used in Drug Development?
In drug development, isomers are used to optimize the therapeutic effects of medications. Researchers can modify the spatial arrangement of molecules to enhance drug efficacy, reduce side effects, or alter the drug’s metabolism within the body. This specificity in drug design is vital for creating effective and safe pharmaceuticals.
Conclusion
The exploration of congeners and isomers illuminates the depth and breadth of chemistry, showcasing the complexity and elegance of molecular structures and their profound implications. These concepts not only enrich our fundamental understanding of chemical processes but also have tangible impacts on various industries, from pharmaceuticals to environmental science and beyond.
By differentiating between congeners and isomers, scientists and researchers can navigate the molecular landscape more effectively, crafting solutions and innovations that harness the unique properties of compounds. This knowledge is pivotal in advancing our ability to develop new drugs, protect the environment, and create products that enhance our lives. The study of these distinctions is a testament to the ongoing quest for understanding that drives the scientific community forward.