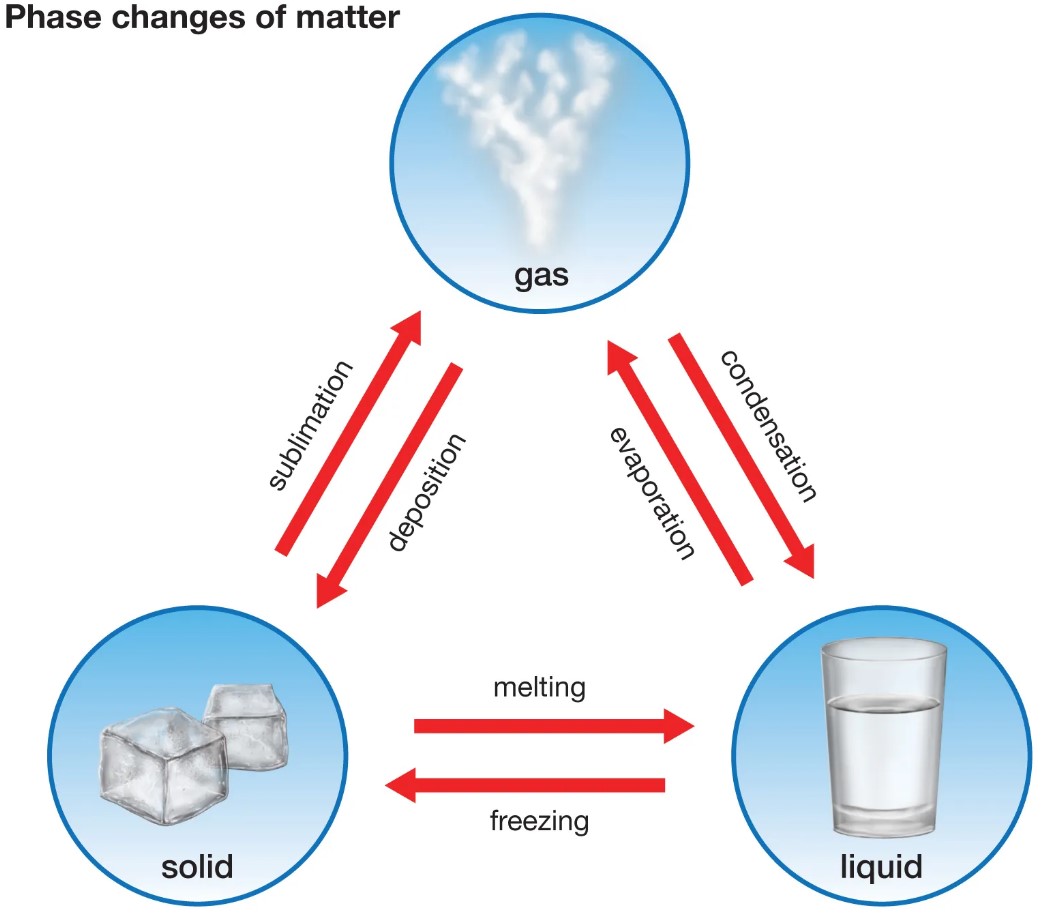
Condensation and freezing are two different processes that involve the changing of a liquid into a solid. The main difference between condensation and freezing is that condensation occurs when vapor in the air is cooled and turned into liquid, while freezing occurs when the temperature of a liquid is reduced to its freezing point and then solidifies. Condensation can occur when warm, humid air comes into contact with a cool surface, like the sides of a cold glass or the outside of a window.
Condensation can occur when warm, humid air comes into contact with a cool surface, like the sides of a cold glass or the outside of a window. Freezing, on the other hand, is a more gradual process that occurs when heat is removed from a liquid until it reaches its solid form. In both cases, the end result is the transformation of liquid into solid.
How condensation and freezing differ
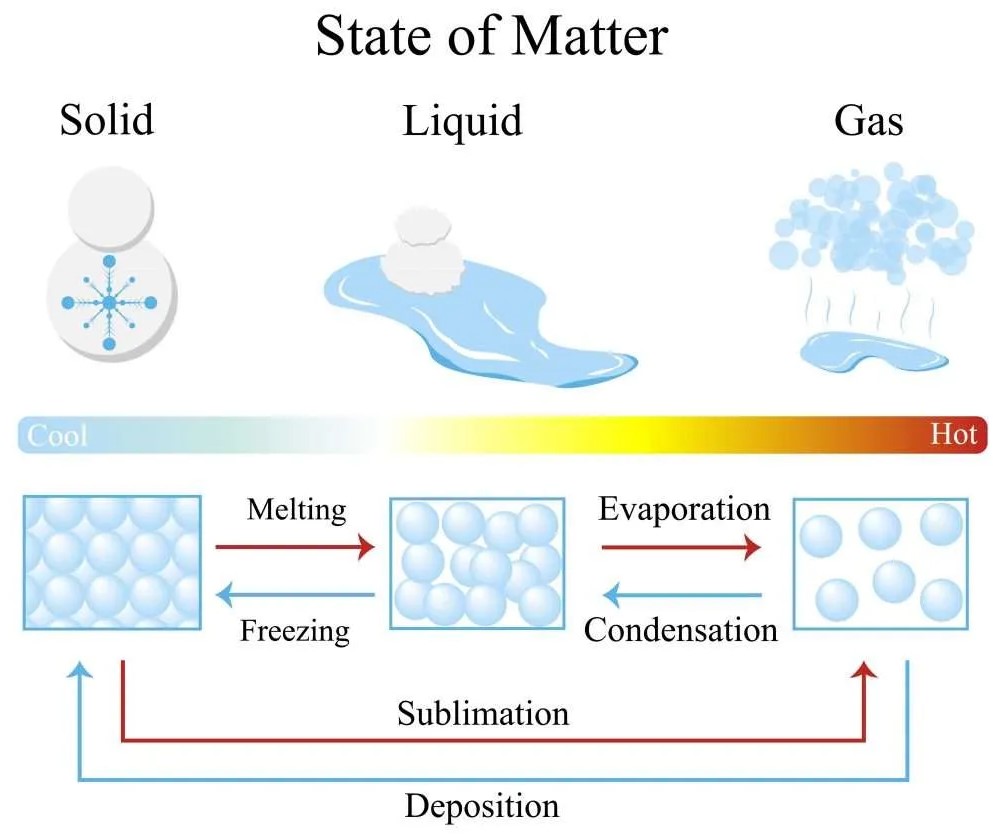
Condensation and freezing are two distinct phases of water. Although these two processes may appear similar, they actually involve different chemical and physical changes.
Condensation occurs when water vapor in the air is cooled and reaches its dew point, while freezing occurs when the temperature of the liquid water drops below 32 degrees Fahrenheit (0 degrees Celsius). Condensation forms small droplets of liquid water, while freezing forms ice crystals.
Both of these processes are important components of the water cycle, and are essential for the maintenance of life on Earth.
Benefits and drawbacks of condensation and freezing
When it comes to condensation and freezing, the two processes have both their benefits and drawbacks. Condensation is the process of converting a gas into a liquid, while freezing is the opposite, taking a liquid and transforming it into a solid. Condensation is a great way to increase the concentration of a liquid, as the vaporized particles collect and become more compact.
Condensation is a great way to increase the concentration of a liquid, as the vaporized particles collect and become more compact. On the other hand, the process of freezing can be used to preserve food and other items for a longer period of time. However, freezing can also lead to the formation of ice crystals, which can cause damage to delicate items.
Both processes have their uses, but it is important to understand the differences between them.
Applications of condensation and freezing
Condensation and freezing are two physical processes that have a wide range of applications. Condensation is the process of a gas turning into a liquid, and freezing is the process of a liquid turning into a solid.
The two processes are quite distinct in terms of their effects on the material, as condensation involves a decrease in temperature while freezing involves an increase in temperature. The differences between the two processes can be further explored through the various applications of condensation and freezing. Condensation is commonly used in the production of alcoholic beverages, in which the process of fermentation converts sugar into an alcoholic gas.
This gas is condensed into a liquid form, producing the desired beverage. Condensation is also used in HVAC systems, where the condensation of the refrigerant is used to cool the air inside the system.
Freezing is used in a variety of applications, most notably in the food industry. Freezing preserves food by reducing the metabolic activity of the food, preventing spoilage and extending its shelf life. Freezing is also used in many industrial processes, such as freeze drying and cryogenic freezing.
Although condensation and freezing are both physical processes, the differences between them can be seen in the various applications they are used for. Condensation is used for cooling and preserving beverages, while freezing is used for preserving food and industrial processes.
Conclusion
In conclusion, the key difference between condensation and freezing is the temperature at which the process occurs. Condensation occurs when water vapor cools and changes into liquid, while freezing occurs when liquid water cools and changes into ice. Both processes occur when the temperature of the air changes, but condensation occurs at a higher temperature than freezing.
Both processes occur when the temperature of the air changes, but condensation occurs at a higher temperature than freezing. Both processes are important for the water cycle and for the maintenance of life on Earth.