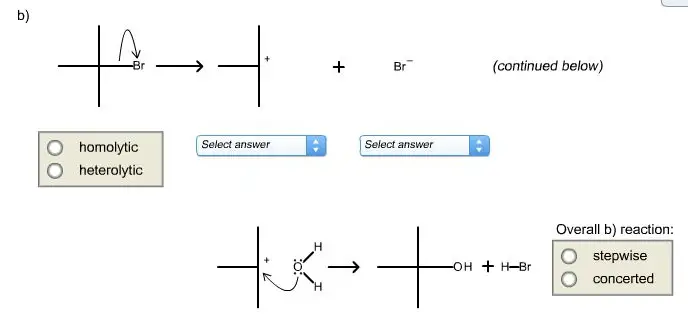
Chemical reactions are fundamental processes that transform substances into different compounds, each with unique properties and applications. These transformations occur through various mechanisms, prominently categorized into concerted and stepwise reactions. Each type of reaction mechanism has distinct pathways and characteristics that influence the behavior and outcome of the chemical processes.
Concerted reactions involve the simultaneous breaking and forming of bonds, resulting in a single-step process without any intermediate stages. Stepwise reactions, on the other hand, occur in multiple stages, each with intermediates and distinct transition states. This fundamental difference affects their speed, selectivity, and conditions under which they are most effectively carried out.
The distinction between these two types of reaction mechanisms is crucial in fields such as synthetic chemistry, pharmaceuticals, and materials science. Understanding their differences aids chemists in selecting the appropriate reaction strategy for synthesizing compounds efficiently and with desired specificity.
Reaction Basics
Definitions: Concerted and Stepwise
In the realm of chemistry, reactions are typically categorized based on the process through which reactants convert to products. Two primary classifications of reaction mechanisms are concerted and stepwise. A concerted reaction is characterized by the simultaneous occurrence of bond-making and bond-breaking steps in a single transition state. This means that the reaction completes in one single step without forming any detectable intermediates.
On the other hand, a stepwise reaction involves multiple stages, each contributing to the overall transformation. These reactions proceed through identifiable intermediates, each stabilized by its own transition state. The formation and decay of these intermediates are key features that define the stepwise nature of these reactions.
Key Reaction Characteristics
Identifying the key characteristics of each type of reaction mechanism is crucial for chemists:
- Speed and selectivity: Concerted reactions tend to be faster as they do not require the stabilization of intermediates. They are often highly selective due to the precise orientation and simultaneous reorganization of molecules.
- Energy profiles: Stepwise reactions often show multiple peaks in their energy diagrams, each corresponding to an intermediate stage, whereas concerted reactions exhibit a single energy barrier.
- Catalytic requirements: Some reactions might require catalysts to proceed, which can influence whether a reaction follows a concerted or stepwise pathway.
Concerted Reactions
Definition and Overview
Concerted reactions are chemical processes where all changes occur in one decisive step. This type of reaction mechanism is often represented in simple and clean energy diagrams with a single peak, reflecting the one-step nature of the process.
Characteristics and Examples
Key characteristics of concerted reactions include:
- Synchroneity: All bonds break and form simultaneously.
- No intermediates: The lack of intermediates makes these reactions cleaner and often easier to predict.
- Examples: A classic example of a concerted reaction is the Diels-Alder reaction, used widely in synthetic organic chemistry to form six-membered rings.
Mechanistic Insights
Mechanistically, concerted reactions are favored under conditions where the energy required to form intermediates is higher than proceeding directly to the product. These reactions are also influenced by the symmetry and orbital alignment of the reactants, crucial for the transition state’s stability.
Stepwise Reactions
Definition and Overview
Stepwise reactions, in contrast to concerted ones, involve multiple discrete steps. Each step has its own transition state and often yields stable intermediates that can sometimes be isolated and characterized.
Characteristics and Examples
Stepwise reactions are characterized by:
- Sequential steps: Each step is distinct and can often be controlled or modified independently.
- Intermediates: These are often key to the reaction and can be targeted for further chemical modifications.
- Examples: An example of a stepwise reaction is the nucleophilic substitution (SN1) reaction, where the departure of a leaving group and the subsequent attack by a nucleophile occur in separate steps.
Mechanistic Insights
The mechanism of stepwise reactions allows for greater control over the reaction process, which can be crucial when synthesizing complex molecules. The energy profile of a stepwise reaction typically shows multiple peaks corresponding to various intermediates and transition states, providing insights into the energy required for each step.
Comparative Analysis
Speed and Complexity
When comparing concerted and stepwise reactions, one of the most striking differences lies in their speed and complexity. Concerted reactions are generally faster because they bypass the need to form stable intermediates. This direct pathway can significantly speed up synthesis, making these reactions particularly useful in high-throughput industrial settings where time is a critical factor.
Conversely, stepwise reactions, with their multiple intermediate stages, are inherently more complex. This complexity can be an advantage in scenarios where the intermediate products are useful or when the reaction conditions need to be finely tuned to achieve a specific outcome. The ability to isolate and manipulate these intermediates provides chemists with the tools to craft intricate molecular architectures that would be challenging or impossible to produce through concerted mechanisms.
Energy Profiles
The energy profiles of reactions provide insights into the stability and feasibility of different pathways:
- Concerted reactions typically display a single energy barrier that must be overcome to reach the product. This single transition state leads to a lower overall energy profile, which is why these reactions can proceed quicker.
- Stepwise reactions show a series of energy barriers corresponding to each intermediate stage. The energy profile of these reactions can appear as a series of peaks and valleys, each peak representing a transition state between stable or semi-stable intermediates.
Understanding these profiles helps chemists predict reaction behavior and choose appropriate catalysts and conditions.
Catalysts and Conditions
Catalysts play a crucial role in modulating the speed and selectivity of both concerted and stepwise reactions. They can lower activation energies, stabilize certain transition states, and increase the overall efficiency of a reaction.
- In concerted reactions, catalysts need to promote a condition where multiple bonds can reorganize simultaneously.
- For stepwise reactions, catalysts might be required to stabilize intermediates or help overcome multiple activation barriers.
The conditions under which a reaction is performed, including temperature, solvent, and pressure, also significantly affect the outcome. Optimal conditions ensure that the desired pathway is favored over possible side reactions.
Practical Implications
Synthetic Chemistry
In synthetic chemistry, the choice between concerted and stepwise reactions can define the success of a synthesis:
- Concerted reactions are preferred when simplicity and speed are paramount. They are particularly valuable in the synthesis of cyclic compounds or when a high degree of atom economy is required.
- Stepwise reactions are indispensable for constructing complex molecules where precise control over each reaction step can lead to better yields and fewer byproducts.
Biological Systems
Understanding reaction mechanisms is not only crucial in synthetic labs but also in biological systems, where enzyme-catalyzed reactions often follow complex pathways. The ability to mimic these processes can lead to the development of new drugs and therapies.
- Enzymes often catalyze stepwise reactions, where control over intermediate formation is crucial for the biological function.
- Insights gained from studying these natural processes often inspire new synthetic methodologies that mimic nature’s efficiency.
Industrial Applications
The implications of choosing the right reaction type extend into industrial applications where scale, safety, and cost-efficiency are critical:
- Concerted reactions might be used in large-scale production processes where the simplicity and speed of the reaction can lead to lower costs and faster production times.
- Stepwise reactions are often preferred in the manufacture of complex organic compounds, such as pharmaceuticals, where precision and the ability to fine-tune reaction conditions are necessary.
Recent Advances
Research Developments
Recent research has led to significant advancements in understanding both types of reactions. Innovative analytical techniques, such as real-time spectroscopy and high-resolution mass spectrometry, provide deeper insights into the mechanistic pathways of complex reactions.
Technological Innovations
Technological innovations, particularly in the field of catalysis and reaction engineering, have revolutionized how chemists approach both concerted and stepwise reactions. New catalysts that are more efficient, selective, and environmentally friendly are continually being developed.
- These advancements not only improve existing processes but also open up new avenues for the synthesis of materials and molecules that were previously thought to be too challenging or inefficient to produce.
Frequently Asked Questions
What are concerted reactions?
Concerted reactions are chemical processes where all bond-breaking and bond-forming events occur simultaneously in a single-step, without the formation of any intermediate compounds. This type of reaction is typically faster and requires specific conditions to proceed efficiently.
How do stepwise reactions work?
Stepwise reactions break down into multiple steps, each with its own transition state and intermediates. This sequential nature allows for greater control over the reaction pathway, which can be crucial in complex synthesis involving multiple reactants.
Why choose a concerted over a stepwise reaction?
Choosing between a concerted and a stepwise reaction depends on factors like the desired yield, the stability of intermediates, and the complexity of the molecular structure being synthesized. Concerted reactions are generally preferred for their speed and simplicity when the conditions allow.
What role do catalysts play in these reactions?
Catalysts can significantly influence both concerted and stepwise reactions by lowering the activation energy required and stabilizing transition states. This can enhance the reaction speed and efficiency, particularly in complex organic syntheses.
Conclusion
The differences between concerted and stepwise reactions underscore the complexity and diversity of chemical processes. Each type of reaction offers unique advantages and limitations, making them suitable for different chemical tasks. Chemists must consider these differences when designing experiments and industrial processes to optimize outcomes and efficiency.
In the pursuit of advancing chemical synthesis, the study of these reaction mechanisms continues to evolve, driven by research that seeks to uncover more effective and sustainable ways to produce chemicals. Understanding these mechanisms not only aids in academic and industrial settings but also enriches the foundational knowledge of chemistry.