Luminescence, the emission of light by a substance not resulting from heat, is a phenomenon that captures the essence of how light and chemistry intersect in fascinating ways. Among its various forms, chemiluminescence and fluorescence stand out for their unique mechanisms and applications, lighting up everything from the depths of the ocean to the intricacies of cellular structures. These processes, though similar in their light-emitting outcomes, are distinct in their origins, mechanics, and uses, painting a vivid picture of nature’s and technology’s ability to harness light.
Chemiluminescence is a chemical reaction that emits light, while fluorescence involves the absorption of light at one wavelength and its re-emission at another, usually longer, wavelength. Essentially, chemiluminescence is powered by chemical energy, whereas fluorescence is driven by the absorption of photons, making them two distinct sides of the luminescence coin. These differences not only define their scientific categorization but also dictate their practical applications, from medical diagnostics to environmental sensing.
The exploration of chemiluminescence and fluorescence reveals a world where light serves as a tool and a signal, offering insights into biological, chemical, and physical processes. Their study not only enriches our understanding of luminescent phenomena but also opens the door to innovative applications across multiple disciplines. As we delve deeper into their characteristics, the distinction between chemiluminescence and fluorescence becomes a cornerstone of advancements in research, technology, and practical applications, reflecting the broader significance of luminescence in science and technology.
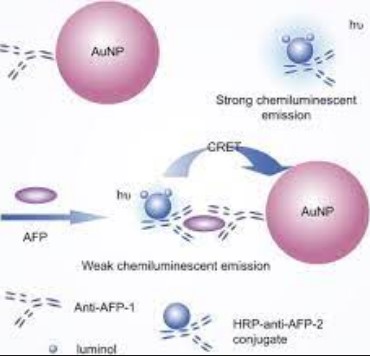
Basic Concepts
Chemiluminescence
Definition and Process
Chemiluminescence is a chemical reaction that results in the emission of light. This process occurs when a chemical reaction provides enough energy to molecules in an excited state, which then release this energy as light when they return to their ground state. Unlike other light-emitting processes, it does not require or emit heat, making it a unique form of luminescence.
The process can be simplified into three main steps:
- Initiation: Reactants undergo a chemical reaction, producing excited intermediates.
- Excitation: These intermediates transfer energy to a luminescent molecule, bringing it to an excited state.
- Emission: The excited molecule returns to its ground state, releasing energy in the form of light.
Applications
Chemiluminescence has a variety of applications, notably in:
- Medical diagnostics: Used in assays and tests for detecting specific proteins or molecules.
- Bioluminescence: Certain organisms, like fireflies and deep-sea creatures, use it to produce light.
- Forensic science: For detecting blood and other substances at crime scenes.
Fluorescence
Definition and Process
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence that occurs almost instantaneously after absorption, with the emitted light having a longer wavelength than the absorbed radiation, resulting in a visible change of color.
The fluorescence process involves:
- Absorption: A photon excites the molecule, elevating it from its ground state to an excited state.
- Non-radiative relaxation: The molecule loses some energy as heat.
- Emission: The molecule returns to its ground state, emitting a photon with less energy (longer wavelength) than the absorbed one.
Applications
Fluorescence is widely used in:
- Imaging and diagnostics: Fluorescent dyes and markers highlight cells and structures in microscopy.
- Sensing and detection: Utilized in sensors to detect environmental pollutants or chemical substances.
- Security: Fluorescent features in currency and documents help prevent counterfeiting.
Key Differences
Energy Sources
The fundamental difference between chemiluminescence and fluorescence lies in their energy sources. Chemiluminescence is powered by the energy from a chemical reaction, while fluorescence relies on the absorption of light or electromagnetic radiation.
Duration
The duration of light emission also distinguishes them. Chemiluminescence can be longer-lasting, as it depends on the duration of the chemical reaction. Fluorescence, however, is nearly instantaneous, ceasing almost as soon as the external light source is removed.
Applications Contrast
In biomedical research, chemiluminescence is favored for its high sensitivity in detecting trace amounts of biological molecules. Fluorescence, with its rapid response and versatility, is invaluable in imaging and diagnostics, providing detailed views of cells and tissues.
Wavelengths and Colors
The spectrum of emitted light further differentiates the two. Chemiluminescence can cover a broad range of wavelengths, often in the visible to near-infrared spectrum. Fluorescence typically exhibits specific wavelengths depending on the fluorophore, leading to bright and distinct colors.
Practical Uses
Chemiluminescence Examples
Bioluminescence in Nature
Many organisms, like jellyfish, fireflies, and certain fungi, exhibit bioluminescence — a form of chemiluminescence. This natural phenomenon serves various purposes, including attraction, camouflage, and communication.
Forensic Science Applications
Chemiluminescence is used in forensic science to detect latent fingerprints and blood traces at crime scenes, providing crucial evidence without damaging samples.
Fluorescence Examples
Fluorescent Dyes and Tags
In biological and medical research, fluorescent dyes and tags enable the visualization of cell structures, proteins, and other molecules under a microscope, aiding in diagnosis and research.
Security and Anti-Counterfeiting Measures
Fluorescent features are incorporated into banknotes, passports, and other secure documents to combat counterfeiting. These features are invisible under normal light but glow under UV light, making them a powerful tool in security.
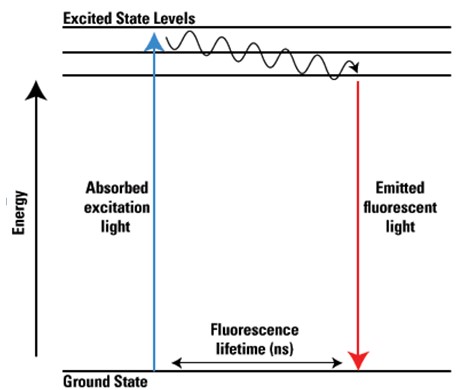
Advantages and Limitations
Chemiluminescence
Advantages in Sensitivity and Detection
Chemiluminescence is highly valued for its exceptional sensitivity and ability to detect low levels of analytes. This characteristic makes it indispensable in fields requiring precise measurements, such as medical diagnostics and environmental monitoring. The process’s high sensitivity ensures that even the most minute quantities of a substance can be identified, providing critical insights in research and practical applications.
Limitations in Control and Intensity
However, chemiluminescence does come with its challenges. One major limitation is the difficulty in controlling the reaction and its intensity. The light emission is directly tied to the chemical reaction’s kinetics, making it harder to standardize and reproduce results across different experiments. This variability can affect the quantitative analysis, especially when precise measurements are crucial.
Fluorescence
Advantages in Versatility and Imaging
Fluorescence stands out for its versatility and the unparalleled quality of imaging it provides. The ability to tag specific molecules with fluorescent dyes allows for detailed visualization of complex biological structures and processes. This versatility extends to a wide range of applications, from medical diagnostics to material science, where fluorescence can illuminate details invisible to the naked eye.
Limitations in Photobleaching and Stability
The primary drawback of fluorescence is photobleaching, the phenomenon where fluorescent molecules lose their ability to emit light after prolonged exposure to the light source. This limits the time that live samples can be observed under the microscope and can degrade the quality of the data collected. Additionally, the stability of fluorescent compounds can be a concern, as they may degrade over time or when exposed to certain environmental conditions.
Technological Innovations
Advancements in Chemiluminescence
Recent years have seen significant advancements in chemiluminescence, particularly in enhancing its sensitivity and application range. Innovations include the development of new chemiluminescent substrates with higher quantum yields, improving the brightness and duration of the emitted light. Technological improvements in detection equipment have also allowed for more precise and accurate measurements, opening new avenues for research and diagnostics.
Enhancements in Fluorescent Techniques
In the realm of fluorescence, technological enhancements have focused on overcoming limitations such as photobleaching and improving the stability of fluorescent dyes. Advances in fluorescent protein engineering have led to the creation of more stable and less photobleachable markers. Additionally, the development of super-resolution microscopy techniques has revolutionized biological imaging, allowing scientists to see structures at the nanoscale with unprecedented clarity.
Environmental and Safety Considerations
Chemiluminescence: Low Environmental Impact
One of the notable advantages of chemiluminescence is its low environmental impact. The process does not require external light sources or high-energy inputs, reducing energy consumption. Moreover, many chemiluminescent reactions use substances that are less harmful to the environment, making it a greener alternative for various applications, including environmental monitoring and sustainable technology development.
Fluorescence: Concerns and Safety Measures
While fluorescence is invaluable in numerous fields, it raises certain environmental and safety concerns. The use of fluorescent dyes and compounds, some of which may be toxic or hazardous, necessitates careful handling and disposal practices. In response, there is a growing emphasis on developing safer, non-toxic fluorescent materials and implementing safety measures to mitigate the impact on both individuals and the environment.
Future Directions
Research Trends in Chemiluminescence
The future of chemiluminescence research looks promising, with trends indicating a move towards increased sensitivity, specificity, and application diversity. Emerging areas of interest include the integration of chemiluminescence with nanotechnology and the development of portable chemiluminescent devices for on-site analysis, which could revolutionize diagnostics, environmental monitoring, and field research.
Innovative Applications of Fluorescence
Innovative applications of fluorescence continue to expand, driven by advancements in materials science, bioengineering, and optical technology. Future directions point towards the development of new fluorescent probes with enhanced stability and specificity, as well as the creation of novel imaging techniques that can provide deeper insights into biological systems and materials. The ongoing evolution of fluorescence technology promises to open new frontiers in science and technology, offering tools that can address some of the most pressing challenges in medicine, environmental science, and beyond.
FAQs
What is Luminescence?
Luminescence refers to the emission of light by a substance that is not a result of heat. This phenomenon encompasses various processes, including chemiluminescence, where light is emitted as a product of a chemical reaction, and fluorescence, where light is absorbed at one wavelength and re-emitted at another. The study of luminescence spans across multiple scientific disciplines, offering insights into the fundamental properties of matter and facilitating numerous applications in biotechnology, medicine, and environmental science.
How Does Chemiluminescence Differ from Fluorescence?
The primary difference between chemiluminescence and fluorescence lies in their sources of energy. Chemiluminescence is the emission of light as a direct result of a chemical reaction, without the need for external light, whereas fluorescence is the result of a material absorbing light at one wavelength and re-emitting it at another. This fundamental difference dictates their respective applications and influences how they are utilized in scientific and technological contexts.
What are the Applications of Chemiluminescence and Fluorescence?
Chemiluminescence and fluorescence find applications across a broad range of fields. Chemiluminescence is widely used in medical diagnostics, forensic science, and environmental monitoring, due to its sensitivity and specificity in detecting trace amounts of substances. Fluorescence, on the other hand, is extensively employed in biological imaging, mineralogy, and the development of fluorescent dyes and markers for research and commercial purposes. Both processes play critical roles in advancing scientific knowledge and technological innovation.
Conclusion
The intricate dance between chemiluminescence and fluorescence illuminates the vast potential of luminescence in science and technology. By emitting light through chemical reactions or by re-emitting absorbed light, these processes not only deepen our understanding of the natural world but also pave the way for groundbreaking applications. From enhancing medical diagnostics to advancing environmental monitoring, the contributions of chemiluminescence and fluorescence are indispensable, marking a luminous path forward in scientific discovery and innovation.
In embracing the complexity and beauty of these luminescent phenomena, we unlock new possibilities and applications that extend beyond the laboratory. The continued exploration of chemiluminescence and fluorescence stands as a testament to the enduring quest for knowledge and the innovative spirit that drives scientific progress. As we venture further into the realm of luminescence, the future shines brightly, illuminated by the endless potential of these fascinating processes.

