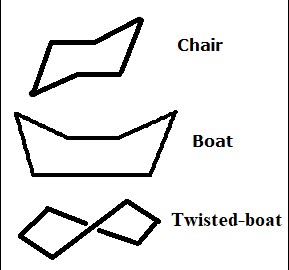
Molecular geometry plays a pivotal role in the realm of organic chemistry, influencing how molecules interact, react, and function in various environments. Among the numerous structural forms, the chair and boat conformations of cyclohexane are especially noteworthy for their distinct properties and implications in chemical reactions. These conformations, representing different spatial arrangements of atoms, are fundamental in understanding the behavior of cyclohexane and other cyclic molecules.
The difference between chair and boat conformations lies primarily in their three-dimensional structure and stability. The chair conformation is more stable due to its ability to minimize torsional and steric strain, allowing atoms to adopt a staggered configuration. Conversely, the boat conformation, with its atoms in a more eclipsed arrangement, faces higher levels of strain, making it less favorable.
This difference is crucial in organic chemistry as it affects the chemical properties and reactivity of molecules. The stability of the chair conformation over the boat conformation has far-reaching implications, from the synthesis of pharmaceuticals to the understanding of biological molecules’ structure and function. Through examining these conformations, chemists can predict reaction outcomes, design more efficient syntheses, and elucidate the behavior of complex organic molecules.
Basic Concepts
Definition of Conformation
In the world of organic chemistry, conformation refers to the unique three-dimensional shape that a molecule can adopt due to the rotation around its single bonds. Unlike isomers, conformers are not separable as they are merely different spatial orientations of the same molecule. This rotation doesn’t break any bonds but significantly affects the molecule’s physical and chemical properties.
Importance of Conformations
Conformations are crucial because they help us understand how molecules interact with each other and their environments. These interactions can influence boiling points, melting points, and reactivity, critical for designing drugs, materials, and industrial chemicals. Recognizing how molecules twist and turn provides insights into their behavior in biological systems and reactions, laying the groundwork for innovation in synthetic chemistry and pharmaceuticals.
Chair Conformation
Description and Features
The chair conformation is a representation of cyclohexane that resembles a chair. It is characterized by its low energy state, making it the most stable and preferred conformation of cyclohexane. This form has alternating axial and equatorial positions, allowing for minimal steric and torsional strain.
Stability Reasons
The chair conformation’s stability comes from its staggered arrangement of hydrogen atoms, reducing repulsive interactions and strain. This efficient packing minimizes the energy of the molecule, making it thermodynamically favorable.
Applications in Chemistry
Understanding the chair conformation has vast applications, especially in synthesizing drugs and elucidating the structure of complex molecules. It aids in predicting reaction outcomes, designing catalysts, and understanding biomolecules’ shapes and functions.
Boat Conformation
Description and Features
In contrast, the boat conformation of cyclohexane looks somewhat like a boat. It is less stable due to eclipsed interactions between hydrogen atoms and inherent steric strain caused by the proximity of atoms on the “bow” and “stern.”
Stability Concerns
The boat form suffers from torsional strain due to eclipsed hydrogen atoms and steric hindrance between atoms at the ends of the molecule. These factors raise its energy level, making it less stable and less common than the chair form.
Applications in Chemistry
Despite its less favorable properties, the boat conformation is still significant in chemistry. It can be an intermediate in reactions where cyclohexane rings change shape, and understanding its properties helps chemists manipulate molecular geometry to achieve desired reaction outcomes.
Comparative Analysis
Energy Levels
The chair conformation is energetically more favorable than the boat conformation due to its ability to minimize strain. The energy difference between these forms is a key consideration in predicting the behavior of cyclohexane and related molecules in chemical reactions.
Steric Strain
Steric strain arises when atoms are forced into close proximity, causing their electron clouds to repel each other. The chair conformation efficiently minimizes this strain by adopting a staggered arrangement of hydrogen atoms, unlike the boat conformation, where atoms are closer, increasing steric strain.
Torsional Strain
Torsional strain is related to the eclipsing of bonds, where atoms are aligned in a manner that increases repulsion. The boat conformation experiences more torsional strain because of its eclipsed hydrogen atoms, contrasting with the chair conformation’s staggered and thus less strained arrangement.
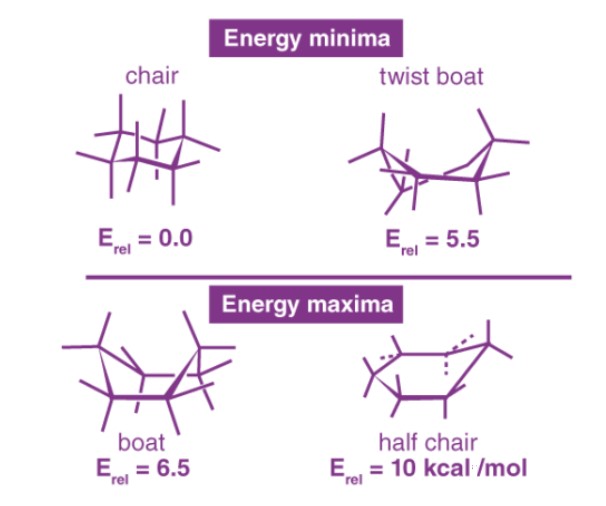
Stability Insights
Factors Affecting Stability
The stability of molecular conformations, such as the chair and boat forms of cyclohexane, is influenced by several key factors. First, steric strain, which is the repulsion between atoms or groups due to spatial constraints, plays a critical role. Second, torsional strain from eclipsed bonds affects stability by increasing internal stress within the molecule. Finally, angle strain, which deviates from the ideal bond angles, can also impact a molecule’s stability.
Role of Substituents
Substituents attached to a cyclohexane ring can drastically affect the molecule’s stability. For instance, bulky groups prefer to occupy the equatorial position in a chair conformation to minimize steric strain. The position and size of these substituents can influence which conformation is more energetically favorable, shifting the equilibrium between different forms.
Visual Differences
Diagrammatic Representations
Understanding the chair and boat conformations of cyclohexane is greatly facilitated by diagrammatic representations. These diagrams visually differentiate the two forms, highlighting the spatial arrangement of atoms and the positions of substituents. Such representations are essential tools in organic chemistry, aiding in the conceptualization of molecular geometry.
Angles and Bonds
The angles and bonds in cyclohexane conformations play a pivotal role in their stability. In the chair conformation, bond angles are close to 109.5 degrees, resembling those in a perfect tetrahedron and minimizing angle strain. The boat conformation, however, has bonds that are slightly distorted, leading to increased strain.
Transition States
Converting Chair to Boat
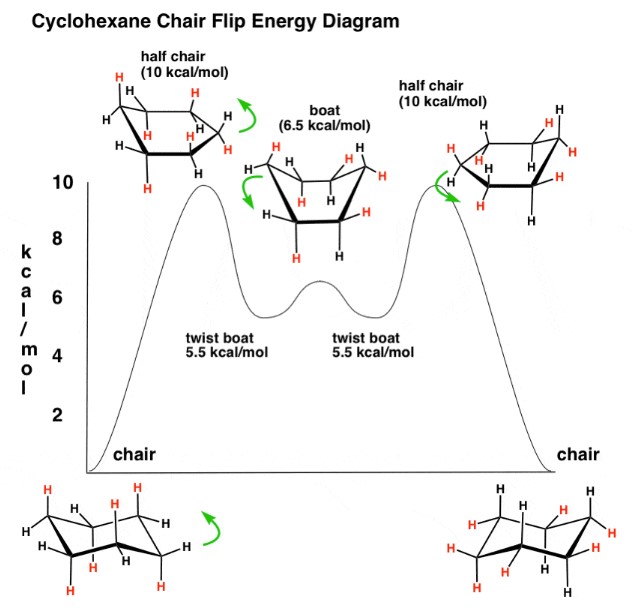
The conversion from chair to boat conformation involves a transition state where the molecule passes through configurations of higher energy. This process is typically induced by thermal energy, which allows the cyclohexane ring to flip from one form to the other, overcoming the energy barrier between the two states.
Energy Barrier Insights
The energy barrier for converting from chair to boat conformation (and vice versa) is a critical aspect of cyclohexane chemistry. This barrier determines how easily and quickly the conversion happens. A higher energy barrier means the conversion is less likely to occur spontaneously at room temperature, keeping the molecule in its more stable form.
Practical Implications
Synthetic Chemistry Applications
The understanding of chair and boat conformations has profound implications in synthetic chemistry. For example, designing synthesis pathways often requires predicting the most stable conformation of intermediates to enhance reaction rates and yields. Furthermore, certain reactions may proceed more favorably with substrates in a specific conformation, guiding the choice of catalysts and conditions.
Biological Relevance
In biology, the shape of molecules like sugars and steroids, which adopt chair or boat conformations, can determine their interaction with enzymes and receptors. This interaction, in turn, influences the molecule’s biological activity and function. Thus, knowledge of these conformations is crucial for drug design, where the active form of a molecule must fit precisely with its biological target to be effective.
FAQs
What is steric strain?
Steric strain occurs when atoms within a molecule are positioned so closely that their electron clouds experience repulsion. This phenomenon is particularly relevant in discussing the boat and chair conformations, where the spatial arrangement of atoms and their substituents can significantly impact a molecule’s overall stability. In the context of cyclohexane conformations, the chair form minimizes steric strain by allowing more room for the substituents, thereby enhancing stability compared to the boat form.
Why is the chair conformation more stable than the boat conformation?
The chair conformation is more stable than the boat conformation due to its ability to minimize both torsional and steric strain. In the chair conformation, hydrogen atoms and substituents are staggered, reducing repulsion between electron clouds and allowing for a more energetically favorable arrangement. This contrasts with the boat conformation, where atoms are more eclipsed, leading to increased strain and, consequently, a less stable structure.
How do chair and boat conformations affect chemical reactions?
Chair and boat conformations can significantly influence the course and outcome of chemical reactions involving cyclohexane and similar cyclic molecules. The spatial arrangement of atoms and substituents in these conformations affects reactivity, with the more stable chair conformation often facilitating reactions under milder conditions. Understanding these conformations allows chemists to predict reaction pathways, design catalysts, and develop synthetic strategies that are more efficient and selective.
Conclusion
The exploration of chair and boat conformations offers profound insights into the intricacies of organic chemistry, illuminating the importance of molecular geometry in determining the stability and reactivity of compounds. Through understanding these conformations, chemists unlock the potential to innovate in the synthesis, design, and application of chemical compounds across a myriad of industries.
As we continue to unravel the mysteries of molecular structures, the knowledge of chair and boat conformations will remain a cornerstone in the development of new pharmaceuticals, materials, and chemical processes. These insights not only advance our scientific endeavors but also enhance our ability to harness the power of chemistry for the betterment of society.