Catenation and allotropy are fundamental concepts in chemistry and material science, shaping how elements behave and interact. While often discussed in academic texts, their practical implications affect everything from the creation of new materials to the stability of various substances used in everyday life. Both phenomena reveal the diversity of element properties under different conditions.
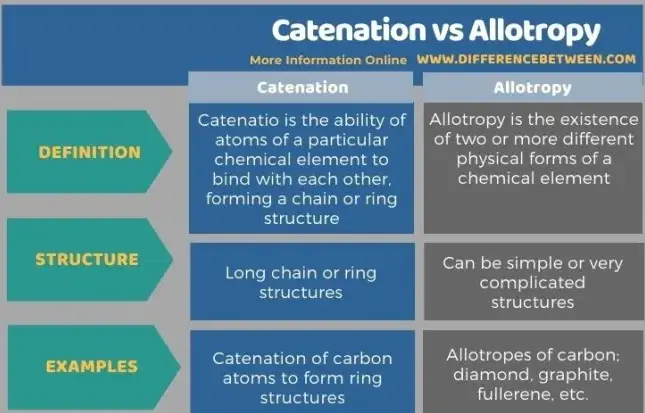
Catenation refers to the ability of an element to form bonds with other atoms of the same element, creating chains or networks. Carbon is the most common example, capable of forming long, stable chains essential for life. Allotropy, on the other hand, describes the existence of an element in two or more different forms, such as the solid allotropes of carbon: diamond and graphite. Each form holds unique properties despite having the same atomic composition.
Understanding these concepts provides a deeper insight into why materials exhibit specific properties and how they can be manipulated to enhance performance or create new materials. For instance, the hardness of diamond and the conductivity of graphite highlight the influence of atomic structure in everyday applications.
Catenation Explained
Definition of Catenation
Catenation is the ability of an atom to form bonds with other atoms of the same element. This unique chemical property allows an element to form a series of bonds that create long chains or complex ring structures. The term originates from the Latin word catena, meaning “chain”.
Chemical Basis of Catenation
The basis for catenation lies in the electronic configuration of an atom. Atoms with a high energy to form covalent bonds are more likely to engage in catenation. These bonds occur when atoms share pairs of electrons, establishing strong links between the same types of atoms. The strength and length of the chains depend on the atom’s ability to provide and share electrons efficiently.
Common Elements Displaying Catenation
Although several elements can form catenated structures, carbon stands out as the most prominent example. Other elements that exhibit notable catenation include:
- Silicon: Forms structures in silicates and silicones.
- Sulfur: Known to form long chains in its elemental state.
- Phosphorus: Can create complex molecules like DNA and ATP in biological systems.
Catenation in Carbon
Unique Properties of Carbon
Carbon’s ability to catenate is fundamental to life on Earth. This element has four electrons in its outer shell, allowing it to form four stable covalent bonds with other carbon atoms or different elements. This flexibility results in a vast array of organic compounds, each with unique properties and functions.
Examples in Organic Compounds
Organic chemistry is rich with examples of catenation. Here are a few:
- Hydrocarbons: Chains of carbon atoms bonded together, surrounded by hydrogen atoms, such as in alkanes, alkenes, and alkynes.
- Ring Compounds: Carbon atoms linked in ring structures, like benzene or cyclohexane.
- Complex Molecules: DNA and proteins, where carbon chains form the backbone.
Allotropy Overview
What is Allotropy?
Allotropy refers to the existence of an element in two or more different forms in the same physical state. These variations, called allotropes, differ in their physical and chemical properties due to variations in the arrangement of the atoms within the element.
Mechanism Behind Allotropy
The phenomenon of allotropy arises from the elements’ ability to adopt different bonding structures. For example, the allotropes of carbon—diamond and graphite—differ because in diamond, each carbon atom forms a strong bond with four other carbon atoms in a tetrahedral structure, resulting in a three-dimensional network. In graphite, each carbon atom bonds tightly to three others in a flat plane, creating layers that easily slide over one another.
Common Allotropes
Allotropes of Carbon
Carbon is an extraordinary element capable of existing in multiple forms, each with distinct properties. The most well-known allotropes of carbon include:
- Diamond: In diamonds, carbon atoms are arranged in a robust three-dimensional lattice where each atom is tetrahedrally bonded to four others. This structure makes diamond the hardest known natural material, with high refractive properties and excellent thermal conductivity.
- Graphite: Graphite has a layered structure, with each layer comprising carbon atoms arranged in a hexagonal lattice. The layers are weakly bonded, allowing them to slide over one another, which makes graphite a good lubricant and an excellent conductor of electricity.
- Fullerenes: These molecules are composed entirely of carbon, taking the form of a hollow sphere, ellipsoid, or tube. Buckyballs and carbon nanotubes are examples of fullerenes, known for their electrical properties and strength.
Other Elemental Allotropes
Other elements also exhibit allotropy, showcasing the versatility and complexity of atomic arrangements:
- Oxygen: Exists as both O₂, which is essential for life, and O₃, known as ozone, which protects the Earth from harmful ultraviolet radiation.
- Sulfur: Found in several allotropic forms, with rhombic and monoclinic sulfur being the most common at room temperature.
- Phosphorus: Exhibits several allotropes, including white, red, and black phosphorus, each differing significantly in reactivity and physical properties.
Catenation vs Allotropy
Key Differences
While both catenation and allotropy involve the properties of elements, their fundamental aspects differ:
- Nature of Bonds: Catenation involves the bonding of an element to itself to form chains or rings, whereas allotropy refers to different physical forms arising from different atomic arrangements.
- Dependence on Element Type: Catenation is prominent in non-metals, particularly carbon, due to their ability to form stable covalent bonds. Allotropy can occur in both metals and non-metals, depending on how atoms are packed or bonded together.
Similarities in Behavior
Despite their differences, catenation and allotropy share a common ground:
- Impact on Material Properties: Both phenomena significantly influence the physical and chemical properties of materials.
- Chemical Flexibility: They highlight the chemical flexibility of elements, showcasing a range of compounds or structures that can be engineered from a single element.
Implications in Science
Impact on Material Properties
The study of catenation and allotropy has profound implications for developing materials with specific properties:
- Strength and Durability: Materials engineered through understanding these phenomena can be made stronger, more durable, or more flexible.
- Electrical and Thermal Conductivity: Alterations in atomic arrangements can enhance or reduce conductivity, making materials suitable for various electronic and thermal applications.
Innovations in Material Engineering
Advances in material science often stem from the exploration of catenation and allotropy:
- Nanotechnology: The use of carbon nanotubes, derived from the catenation and allotropic properties of carbon, has revolutionized the development of stronger yet lighter materials.
- Semiconductors: Silicon’s ability to catenate has been essential in developing the semiconductor industry, crucial for all modern electronics.
- Renewable Energy: Materials like graphene, a single layer of graphite, are being studied for their exceptional electrical properties and potential use in energy storage solutions.
Frequently Asked Questions
What is Catenation?
Catenation is the chemical property of an element to bond with other atoms of the same type, forming chains or complex structures. This property is most prominently seen in carbon, which forms the backbone of all organic molecules.
How does Allotropy Occur?
Allotropy occurs when an element exists in two or more different physical forms in the same physical state. These variations arise due to different arrangements of atoms within the element, significantly affecting its chemical properties.
Differences Between Catenation and Allotropy?
While both involve elements, catenation is about forming bonds with similar atoms, whereas allotropy pertains to different physical forms of the same element. Understanding both is crucial for studying chemical behavior and material science.
Examples of Materials Affected by Catenation?
Materials like polymers and various organic compounds exhibit properties influenced by catenation. For example, the versatility of plastics and rubbers is largely due to the catenation of carbon atoms.
Why is Allotropy Important in Materials Science?
Allotropy explains why materials like graphite and diamond differ so drastically despite being composed of the same element, carbon. This knowledge is essential for applications requiring specific material properties, like hardness or electrical conductivity.
Conclusion
The exploration of catenation and allotropy unravels the complexity and versatility of elements, particularly carbon. These concepts not only enhance our understanding of chemical bonds and structures but also pave the way for innovations in material science and engineering. By manipulating these properties, scientists and engineers can tailor materials to meet specific needs, driving advancements in technology and industry.
In summary, catenation and allotropy serve as the foundation for developing materials with desired traits. The continued study of these properties is crucial for future technological developments, highlighting the importance of chemistry in solving real-world problems and enhancing our quality of life.