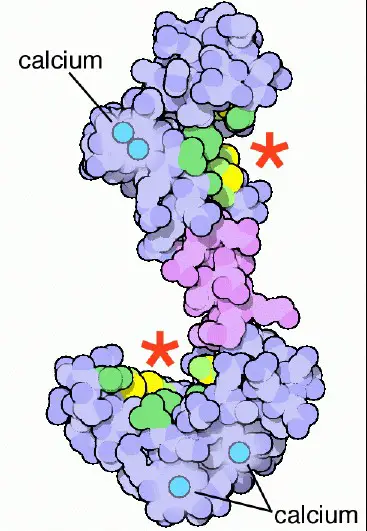
Calcium ions (Ca2+) play a pivotal role in various cellular processes, acting as a universal signal in numerous biological systems. This signaling is intricately regulated by calcium-binding proteins, among which Calmodulin and Troponin C are paramount. These proteins facilitate a range of functions, from muscle contraction to enzyme regulation, showcasing the versatility of calcium signaling in cellular activities.
Calmodulin and Troponin C, despite their shared role in calcium-binding, serve distinct functions within the cell. Calmodulin acts as a multifunctional intermediary protein, modulating various enzymes, ion channels, and other proteins. In contrast, Troponin C plays a specific role in muscle contraction, being an integral component of the troponin complex in skeletal and cardiac muscles. This fundamental difference underscores the specialized roles these proteins play in maintaining cellular health and function.
Delving into the molecular intricacies, Calmodulin and Troponin C exhibit unique structural characteristics that enable their specific functions. Calmodulin is known for its flexibility, allowing it to interact with a wide range of target proteins. On the other hand, Troponin C, while sharing structural similarities with Calmodulin, features unique adaptations that tailor it for its critical role in muscle contraction. This distinction not only highlights the evolutionary finesse of cellular machinery but also emphasizes the complexity of calcium-mediated signaling pathways.
Calmodulin Overview
Role in Cells
Calmodulin (CaM) is a crucial calcium-binding messenger protein involved in a myriad of cellular functions. Its role spans signal transduction mechanisms, where it interprets calcium signals triggered by various physiological stimuli.
Signal Transduction Mechanisms
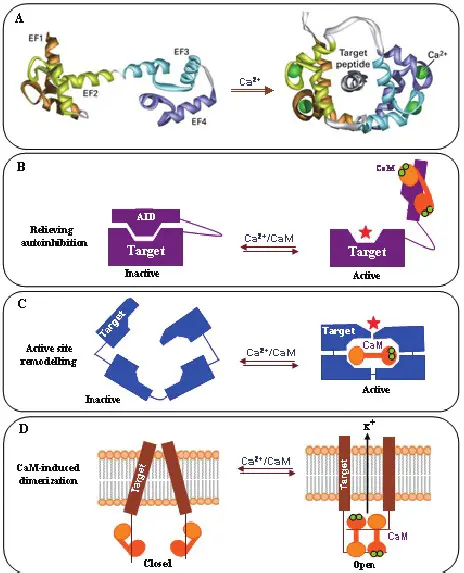
Calmodulin serves as a pivotal intermediary in calcium signaling pathways. When calcium levels rise in the cell, CaM binds to these ions, undergoing a conformational change that allows it to interact with target proteins. This interaction is fundamental in transmitting signals inside the cell, leading to various cellular responses.
Activation of Enzymes and Ion Channels
The interaction between CaM and its targets can activate or inhibit enzymes and ion channels, thus regulating cellular activities. For example, CaM activates kinases and phosphatases, enzymes that play roles in cell metabolism, gene expression, and cell cycle progression. Additionally, it controls the opening and closing of ion channels, which affects neurotransmitter release, muscle contraction, and other vital processes.
Structure
Calmodulin’s structure is uniquely suited to its role as a flexible signal transducer.
Consists of Two Globular Domains
CaM features two distinct globular domains, connected by a flexible linker. Each domain can bind two calcium ions, allowing CaM to bind up to four calcium ions simultaneously. This structure enables the protein to change shape and interact with a wide range of target molecules.
Flexibility and Binding Mechanism
The flexibility of CaM, facilitated by its central linker, allows it to wrap around target proteins effectively. This dynamic binding mechanism is crucial for its ability to regulate diverse cellular functions.
Troponin C Overview
Function in Muscle Contraction
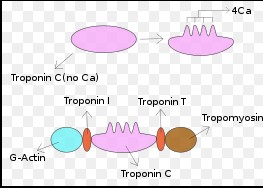
Troponin C (TnC) plays a specific role in muscle function, acting as a vital component of the troponin complex, which regulates muscle contraction in both skeletal and cardiac muscles.
Key Component of the Troponin Complex
The troponin complex, consisting of troponin I, T, and C, works together with tropomyosin to regulate the interaction between actin and myosin. TnC, in particular, binds calcium, initiating the cascade of events that lead to muscle contraction.
Regulation of Skeletal and Cardiac Muscle Contraction
In skeletal muscles, TnC’s binding to calcium causes a shift in the troponin complex, allowing myosin to bind to actin and initiate contraction. In cardiac muscles, this mechanism is finely tuned to control heartbeats, demonstrating TnC’s critical role in muscle function across different muscle types.
Structure
While sharing a basic structure with CaM, TnC has unique features that tailor it for its role in muscle contraction.
Similarities to Calmodulin
Like CaM, TnC possesses EF-hand motifs for calcium binding. However, its structural adaptation emphasizes its specialized function in muscle cells.
Unique Features for Muscle-specific Functions
TnC’s structure is optimized for its role in the troponin complex. Its calcium-binding sites have a high affinity, appropriate for the rapid response required for muscle contraction. This specialization underlines the protein’s essential role in muscle physiology.
Comparative Analysis
Calcium-Binding Sites
The calcium-binding sites of CaM and TnC are critical to their functions but differ in number, location, and impact on their respective functions.
Number and Location
CaM can bind up to four calcium ions, two in each globular domain. TnC, while also possessing EF-hand motifs, has sites specifically adapted for muscle contraction signaling.
Impact on Function
The binding of calcium to these proteins triggers different cellular events. For CaM, it means activation of various enzymes and channels, while for TnC, it specifically triggers muscle contraction.
Activation Mechanism
Differences in the activation mechanisms of CaM and TnC reflect their specialized roles within the cell.
Differences in Targets and Pathways
While CaM interacts with a wide range of proteins across various pathways, TnC specifically targets the troponin complex in muscle cells. This specialization highlights the proteins’ distinct roles in cellular signaling and muscle contraction.
Structural Flexibility
Structural flexibility allows these proteins to bind to calcium and undergo necessary conformational changes to activate their functions.
Conformational Changes Upon Calcium Binding
Both CaM and TnC undergo significant conformational changes upon calcium binding. However, the specific changes and their impacts differ, with CaM facilitating varied cellular responses and TnC focusing on muscle contraction regulation. This comparative analysis underlines the tailored functions of Calmodulin and Troponin C in their respective domains, showcasing the elegance of cellular mechanisms in response to calcium signaling.
Physiological Roles
Calmodulin
Calmodulin (CaM) is a master regulator in cells, orchestrating a wide array of physiological processes. Its ability to bind calcium and interact with various cellular targets makes it essential in numerous signaling pathways.
Diverse Cellular Processes
- Neurotransmitter Release: CaM plays a pivotal role in the release of neurotransmitters, the chemicals that transmit signals across a synapse from one neuron to another. It regulates the activity of enzymes and ion channels that are crucial for this process.
- Metabolism Regulation: CaM is integral in metabolic pathways, influencing the activity of metabolic enzymes. It responds to calcium levels to regulate energy production and consumption, ensuring cellular energy demands are met efficiently.
Troponin C
Troponin C (TnC), while sharing the calcium-binding property with CaM, has a more specialized role in the body, primarily focusing on muscle contraction.
Muscle Contraction Specificity
- Skeletal Muscles: In skeletal muscles, TnC’s role is to initiate contraction by binding calcium and triggering the troponin complex to interact with actin and myosin filaments. This process is fundamental for voluntary movements.
- Cardiac Muscles: The function of TnC in cardiac muscles is critical for heartbeats. It ensures that heart contractions are precisely regulated, maintaining the rhythm and strength necessary for blood circulation.
Clinical Significance
The roles of CaM and TnC in cellular functions translate to significant implications in health and disease, making them important in clinical settings.
Diagnostics and Prognostics
Biomarkers in Cardiac Diseases
- Troponin C is a key biomarker for diagnosing heart attacks and other cardiac conditions. Elevated levels of cardiac-specific TnC isoforms in the blood are indicative of myocardial injury, offering a reliable tool for early diagnosis and management of cardiac diseases.
Therapeutic Targets
The unique roles of CaM and TnC in cellular processes make them promising targets for drug development.
- Drug Development: Researchers are exploring inhibitors and modulators of CaM and TnC as potential treatments for various conditions, including cardiovascular diseases, neurological disorders, and cancers. By targeting these proteins, therapies can be designed to modulate their activity, offering new avenues for treatment.
Evolutionary Perspective
The existence and specialization of CaM and TnC highlight their importance in the evolutionary history of life.
Evolutionary Origins and Divergence
- Origins: Calmodulin and Troponin C share a common ancestor with other EF-hand calcium-binding proteins. This family of proteins has evolved over millions of years, adapting to the complex needs of cellular signaling and function.
- Divergence: The divergence between CaM and TnC reflects their adaptation to specific cellular roles. While CaM evolved to regulate a broad range of cellular functions across different tissues, TnC specialized in muscle contraction, a critical function for mobility and survival.
Adaptive Significance in Cellular Functions
- Adaptation to Cellular Needs: The evolution of CaM and TnC underscores their adaptive significance. CaM’s versatility allows cells to respond to various signals and stimuli, while TnC’s specificity ensures efficient muscle contraction, vital for movement and heart function.
- Contribution to Organismal Survival: The roles of CaM and TnC are fundamental in physiological processes that contribute to the survival and reproduction of organisms. Their evolution reflects the fine-tuning of cellular mechanisms to meet environmental challenges and opportunities.
Frequently Asked Questions
How do Calmodulin and Troponin C bind calcium?
Calmodulin and Troponin C bind calcium ions through their EF-hand motifs, a helix-loop-helix structure conducive to calcium binding. Calmodulin has four such sites, allowing for versatile binding and regulation of multiple targets. Troponin C, adapted for muscle function, also possesses these motifs but is optimized for the rapid and regulated response necessary for muscle contraction.
What is the significance of Calmodulin in cellular signaling?
Calmodulin is a crucial mediator in cellular signaling, involved in processes ranging from cell division and metabolism to memory formation. By interacting with a variety of target proteins, it acts as a key regulator of cellular responses to calcium changes, adjusting cellular activities to meet physiological demands.
How does Troponin C contribute to muscle contraction?
Troponin C is integral to the mechanism of muscle contraction. It binds calcium ions during muscle activation, inducing a conformational change in the troponin complex. This change enables the interaction of actin and myosin, the fundamental process driving muscle contraction, thereby playing a direct role in the regulation of muscular force and movement.
Why are Calmodulin and Troponin C important for medical research?
Both proteins are significant in medical research due to their involvement in critical cellular processes and diseases. Calmodulin’s role in signal transduction makes it a target for studying neurological disorders and cancer. Troponin C, particularly its cardiac-specific isoform, is a biomarker for heart conditions, making it crucial for diagnostics and therapeutic research.
Conclusion
The roles of Calmodulin and Troponin C in cellular operations underline the complexity and specificity of calcium signaling in biological systems. Calmodulin’s wide-reaching influence across various cellular pathways, alongside Troponin C’s specialized function in muscle contraction, exemplifies the evolutionary optimization of these proteins for their respective roles. Such distinctions not only illuminate the intricate dance of molecular interactions governing cellular life but also pave the way for targeted medical interventions and therapies.
Understanding these proteins’ unique characteristics and functions offers a glimpse into the cellular machinery’s precision, where every component plays a critical role in sustaining life. As research continues to unravel these proteins’ mysteries, it holds the promise of harnessing their potential for advancing human health, marking a continuing journey into the microscopic world that orchestrates our macroscopic existence.