Calcium sulfate and plaster of Paris are two materials frequently used across various industries, each possessing unique properties and applications. While they share a common chemical origin, their distinct characteristics set them apart in both functionality and usage. This differentiation is critical for professionals in fields ranging from construction to medicine, where choosing the right material can significantly impact the quality and efficacy of a project or treatment.
Calcium sulfate is a naturally occurring mineral known for its versatility and various forms, while plaster of Paris is a quick-setting gypsum plaster consisting of a fine white powder, which hardens when moistened and allowed to dry. The primary difference between the two lies in their chemical structure and physical properties. Calcium sulfate serves as the base mineral that, when heated and ground, transforms into the more refined and rapid-setting plaster of Paris.
In the context of practical applications, these materials are not interchangeable. Calcium sulfate is often utilized for its chemical properties in industrial settings, whereas plaster of Paris is favored for its moldability and quick setting time, making it ideal for casts and molds. Understanding their distinct characteristics enables professionals to make informed decisions that optimize the outcomes of their specific projects.
Calcium Sulfate Overview
Definition and Forms
Calcium sulfate is a white crystalline solid, commonly found in both its hydrated and anhydrous forms in nature. The chemical formula for this compound is CaSO4. It is widely recognized for its abundance in the Earth’s crust and can be mined from natural sources such as gypsum and anhydrite.
Chemical Composition
The chemical structure of calcium sulfate is straightforward, comprising one calcium atom, one sulfur atom, and four oxygen atoms. This simple composition lays the foundation for its diverse applications across various industries.
Different Forms and Hydrates
Calcium sulfate occurs in several forms and hydrates, the most common being:
- Gypsum (Calcium Sulfate Dihydrate): Chemically expressed as CaSO4·2H2O, gypsum contains two molecules of water. It is the most prevalent form used in building materials like drywall.
- Anhydrite (Anhydrous Calcium Sulfate): As CaSO4, it lacks water molecules, making it denser and harder than gypsum. Anhydrite is used in larger-scale industrial applications requiring materials with minimal water content.
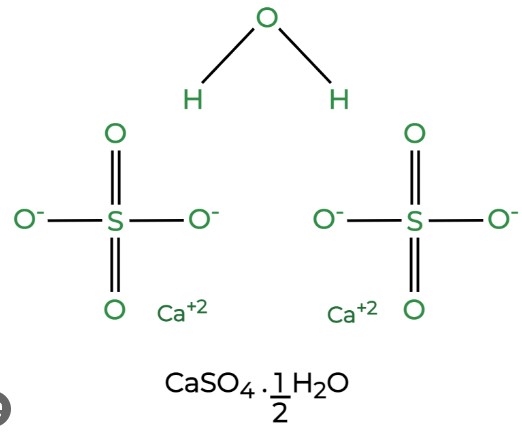
- Hemihydrate (Plaster of Paris): When gypsum is partially dehydrated, it forms CaSO4·0.5H2O, known as plaster of Paris.
Properties
Physical Characteristics
Calcium sulfate’s physical properties vary depending on its hydration level. Gypsum is relatively soft, which facilitates its use in construction, whereas anhydrite is harder and more suitable for high-load applications. The color can range from white to light grey.
Chemical Properties
The chemical stability of calcium sulfate allows it to perform well in various environmental conditions, with a notable resistance to erosion and degradation. This stability, coupled with its non-toxic nature, makes it a preferred choice for food additives and industrial uses.
Plaster of Paris Overview
Definition and Preparation
Plaster of Paris is a fine, white powder primarily used for making sculptures, molds, and casts. It is derived from gypsum, which undergoes a process of heating and then grinding.
How it is Derived from Calcium Sulfate
The transformation from gypsum (calcium sulfate dihydrate) to plaster of Paris (calcium sulfate hemihydrate) involves:
- Heating Gypsum: Typically, the gypsum is heated to about 150°C. At this temperature, the material loses water molecules bound within its crystal structure.
- Processing into Powder: Once the heating process is complete, the hemihydrate is then ground into a fine powder, known as plaster of Paris.
Manufacturing Process
The manufacturing of plaster of Paris is carried out through the following steps:
- Extraction and Crushing: Raw gypsum is extracted from mines and crushed into smaller pieces.
- Heating: The crushed gypsum is then heated in kettles or rotary kilns at controlled temperatures.
- Milling: After heating, the material is milled to a fine powder and packaged for distribution.
Properties
Physical Characteristics
Plaster of Paris is known for its smoothness and fine texture, making it an ideal choice for detailed artistic work. It starts as a dry powder and, when mixed with water, turns into a moldable paste.
Setting and Hardening
One of the most critical properties of plaster of Paris is its ability to set and harden rapidly. When water is added to the powder:
- Initial Set: The mixture starts to harden within minutes, allowing for quick creation of molds.
- Final Hardening: The final hardening process takes place over a few hours, during which the material becomes solid and durable.
Key Differences
Chemical Structure
Comparison of Molecular Makeup
Calcium sulfate and plaster of Paris differ fundamentally in their molecular composition. Calcium sulfate, in its most common form as gypsum, consists of one molecule of calcium sulfate attached to two molecules of water (CaSO4·2H2O). In contrast, plaster of Paris is the hemihydrate form of calcium sulfate, which includes one molecule of calcium sulfate bonded to half a molecule of water (CaSO4·0.5H2O).
This difference in water content is not merely quantitative but impacts the properties and applications of each substance profoundly. The removal of water through controlled heating transforms gypsum into plaster of Paris, altering its molecular structure and thereby its usability.
Physical Properties
Differences in Texture, Color, and Solubility
- Texture: Plaster of Paris is finer and smoother compared to the more granular texture of gypsum-derived calcium sulfate.
- Color: Both materials are generally white, but plaster of Paris can appear more brilliant due to its finer particle size and higher purity.
- Solubility: Calcium sulfate in its dihydrate form (gypsum) is slightly more soluble in water than the hemihydrate form (plaster of Paris), influencing how each reacts to moisture and humidity.
Uses and Applications
Industrial and Medical Applications for Each
- Calcium Sulfate:
- Industrial Uses: It is used in the manufacture of Portland cement and as a soil conditioner. Its properties help improve soil structure and reduce compaction, leading to better water penetration and root growth.
- Medical Uses: Calcium sulfate is used as a plaster for setting bones and as a tablet excipient in the pharmaceutical industry.
- Plaster of Paris:
- Industrial Uses: Widely used in the production of decorative plasterwork and architectural elements. It is also used in creating molds for ceramics and as a mold material in the manufacturing of glass.
- Medical Uses: Predominantly used for making smooth, contouring casts to stabilize broken bones. Its quick setting time and ease of use make it ideal for emergency settings.
Handling and Safety
Safety Measures and Precautions
Handling both calcium sulfate and plaster of Paris requires certain precautions to ensure safety, particularly in industrial environments:
- Dust Control: Both materials can generate fine dust, which is harmful if inhaled. Using dust suppression systems and personal protective equipment like masks is essential.
- Moisture Control: As these materials react with water, it is crucial to keep them dry during storage to prevent premature setting or degradation.
- Equipment Safety: When working with heated equipment for processing gypsum into plaster of Paris, appropriate safety measures must be in place to prevent burns or other injuries.
Advantages of Each
Benefits of Calcium Sulfate
Industrial Uses
Calcium sulfate’s role in cement production enhances the final product’s durability and quality. It acts as a natural fire retardant in gypsum board, providing additional safety in building construction.
Environmental Benefits
Being a naturally occurring mineral, calcium sulfate contributes to sustainable practices. It is biodegradable and non-toxic, making it a favorable choice for eco-friendly applications.
Benefits of Plaster of Paris
Artistic and Medical Uses
Plaster of Paris has been a staple in art and medicine due to its moldability and fine texture which allows for detailed and precise works. It is particularly valued in the arts for creating sculptures and in medicine for making smooth, supportive casts.
Ease of Use and Versatility
The simplicity and versatility of plaster of Paris make it popular across various fields. It can be easily mixed and shaped, sets quickly, and can be painted or modified to suit different needs, from classroom projects to intricate architectural details.
FAQs
What is calcium sulfate?
Calcium sulfate is a salt that occurs abundantly as gypsum. Its anhydrous form is used in various industrial processes, including as a desiccant. It is valued for its properties such as non-toxicity, stability, and ability to act as a moisture regulator.
How is plaster of Paris made?
Plaster of Paris is produced by heating calcium sulfate dihydrate, or gypsum, to about 150 degrees Celsius. This process drives off part of the water, resulting in the powdery product that, when mixed with water, reforms into gypsum but in a hardened form.
Can plaster of Paris be reused?
Once plaster of Paris has set and hardened, it cannot be re-moistened or remolded. This characteristic makes it essential to mix only as much material as needed for immediate use.
What are the main uses of calcium sulfate?
Calcium sulfate is used in a variety of applications, including as an additive in foods, a coagulant in the production of tofu, and a soil conditioner in agriculture. It is also used in the dental and pharmaceutical industries as an impression material.
Is plaster of Paris environmentally friendly?
Plaster of Paris is considered environmentally friendly due to its natural origin and biodegradability. However, its manufacturing process is energy-intensive, which can be mitigated by sourcing from responsible manufacturers that adhere to sustainable practices.
Conclusion
Calcium sulfate and plaster of Paris, while closely related, serve distinct purposes across different sectors due to their inherent chemical and physical properties. Recognizing these differences is crucial for selecting the appropriate material for specific tasks, ensuring efficiency and effectiveness in professional outcomes.
By fully leveraging the unique qualities of each substance, professionals can optimize their use in everything from construction projects to artistic endeavors, contributing to more refined, durable, and practical results. This knowledge not only facilitates better material choices but also enhances the sustainability and quality of the final products.

