Calcination and pyrolysis are two fundamental processes widely used in various industrial sectors to alter the properties of materials through thermal treatment. While both involve the application of heat, their purposes, conditions, and outcomes differ significantly. These processes are pivotal in materials engineering, environmental management, and energy production, playing crucial roles in the manufacturing of essential products.
Calcination involves heating a substance in a controlled environment to a high temperature, below its melting point, causing thermal decomposition or phase transition without melting. This process is commonly employed to remove volatile substances, induce thermal decomposition, or cause phase transitions in materials like limestone, turning it into lime. Pyrolysis, on the other hand, is the thermal decomposition of materials at elevated temperatures in an inert atmosphere, typically leading to the breakdown of chemical bonds and resulting in the formation of various byproducts, including char, oil, and gas.
The significance of these processes extends beyond mere heat treatment; they are integral to the creation of building materials, energy resources, and even waste management solutions. By understanding their distinct mechanisms and applications, industries can optimize their use, enhancing efficiency and sustainability.
Basic Concepts
Calcination Defined
Definition and Basics
Calcination is a thermal process commonly used in various industrial sectors to alter the properties of substances by heating them to high temperatures in a controlled environment. This process does not melt the material but typically causes decomposition or a phase transition. It is essential in industries that require the alteration of physical or chemical properties of materials without reaching their melting points.
Common Uses in Industry
Calcination has widespread applications in industries such as:
- Cement production: where limestone (calcium carbonate) is converted into lime (calcium oxide), a critical ingredient in cement.
- Metal extraction: particularly in the purification and refinement of metals from ores.
- Glass manufacturing: where it helps in decomposing raw materials like sand into simpler compounds ready for fusion.
- Plaster production: where gypsum is heated to form plaster of Paris.
Pyrolysis Explained
Definition and Principles
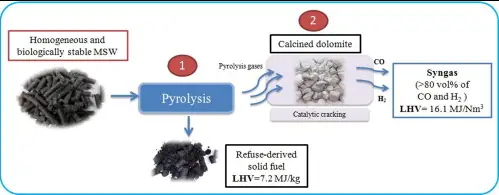
Pyrolysis is a process of chemically decomposing organic materials at elevated temperatures in the absence of oxygen. Unlike combustion, pyrolysis does not involve the complete oxidation of the material but instead breaks down complex substances into simpler compounds, which can be both volatile and non-volatile.
Key Applications
Pyrolysis is crucial in:
- Biofuel production: converting biomass into bio-oil, syngas, and charcoal, which are excellent sources of renewable energy.
- Waste management: transforming discarded plastics and organic waste into usable products, reducing landfill use and pollution.
- Charcoal production: a less pollutive way to produce charcoal compared to traditional methods.
- Chemical industry: as a method for producing raw materials for various chemicals.
Process Characteristics
Temperature Requirements
Heat Levels for Calcination
The temperatures for calcination typically range from 500°C to 1200°C, depending on the material being processed. For example:
- Limestone calcination: occurs at about 900°C to produce lime.
- Kaolin: used in ceramics, is calcined at around 750°C to improve its whiteness and alter its physical properties.
Heat Levels for Pyrolysis
Pyrolysis temperatures are generally higher than those for calcination, often between 400°C and 1400°C. The specific temperature depends on the desired product:
- Low-temperature pyrolysis (around 400°C-600°C): ideal for maximizing liquid bio-oil production.
- High-temperature pyrolysis (up to 1400°C): focuses on producing a larger proportion of syngas and solid residue (char).
Atmospheric Conditions
Oxygen Presence in Calcination
Calcination typically takes place in an oxidizing or neutral atmosphere, where limited oxygen is involved but not enough to cause combustion. This controlled oxygen presence is crucial to ensure that the material undergoes a chemical reaction without burning.
Oxygen Absence in Pyrolysis
The absence of oxygen is a defining characteristic of pyrolysis, preventing the material from combusting while allowing it to decompose into simpler chemical substances. This inert atmosphere is essential to produce the char, oils, and gases typical of pyrolysis.
Material Transformations
Changes in Calcination
Chemical Transformations
During calcination, chemical changes include the release of carbon dioxide from carbonates and the breakdown of hydroxides and sulfates. These transformations are critical for producing pure and functional materials for various industrial applications.
Physical Changes
Physical changes might include increased porosity and changes in color or texture, which are important for the material’s final use, such as enhanced reactive surface area in catalysts.
Changes in Pyrolysis
Breakdown of Organic Materials
Pyrolysis leads to the thermal degradation of organic materials, breaking down long molecular chains into shorter ones. This process results in the production of a variety of smaller molecules, which form the basis of bio-oil and syngas.
Resultant Byproducts
The byproducts of pyrolysis include:
- Char: a solid residue rich in carbon and inorganic compounds.
- Bio-oil: a dense, energy-rich liquid that can be further refined or directly burned for energy.
- Syngas: a mixture of hydrogen, carbon monoxide, and often some carbon dioxide, used directly as fuel or as a precursor for other chemical products.
Equipment and Technology
Tools for Calcination
Types of Calciners
Calciners are industrial equipment used in the calcination process. The major types include:
- Rotary kilns: Rotate the material, allowing for continuous processing and uniform heat distribution.
- Shaft kilns: Stand vertically, often used for lime production because of their energy efficiency.
- Fluidized bed calciners: Use a fluidized bed to enhance heat transfer and reaction rates, ideal for fine powders.
Technological Advancements
Recent advancements in calciner technology focus on improving energy efficiency and reducing emissions. These include:
- Automated control systems that optimize temperature and processing times.
- Development of alternative fuel systems that can use biomass or waste materials.
- Enhanced insulation materials to reduce heat loss.
Tools for Pyrolysis
Pyrolysis Reactors
Key types of pyrolysis reactors include:
- Fixed-bed reactors: Simple design, suitable for small-scale operations.
- Fluidized-bed reactors: Offer better heat transfer and faster reaction times for continuous large-scale operations.
- Rotating cone reactors: Used for rapid pyrolysis to produce high yields of bio-oil.
Innovations in Technology
Innovative developments in pyrolysis technology are aimed at enhancing bio-oil quality and yield. These innovations include:
- Catalytic pyrolysis: Introduces catalysts to improve oil quality and reduce acid content.
- Microwave-assisted pyrolysis: Uses microwave energy for rapid heating, which can significantly decrease process time and improve energy efficiency.
Environmental Impact
Emissions from Calcination
Types of Emissions
Calcination can emit several types of pollutants:
- Carbon dioxide (CO2): Especially from the decomposition of carbonates.
- Sulfur dioxide (SO2) and nitrogen oxides (NOx): From the burning of fossil fuels used in the process.
Impact on Environment
The environmental impact of these emissions includes:
- Contribution to global warming due to CO2 emissions.
- Acid rain potential from SO2 and NOx emissions.
- Air quality degradation, affecting human health and ecosystems.
Emissions from Pyrolysis
Comparison with Calcination
Pyrolysis generally produces fewer direct emissions than calcination because it operates in an oxygen-free environment, reducing the formation of NOx and SO2.
Reduction Strategies
Strategies to reduce emissions from pyrolysis include:
- Using scrubbers and filters to clean exhaust gases.
- Energy recovery systems to utilize the heat produced during pyrolysis.
- Feedstock pre-treatment to reduce impurities that can lead to emissions.
Economic Aspects
Cost Factors
Operating Costs of Calcination
Major cost factors for calcination include:
- Fuel consumption: High temperatures require substantial energy inputs.
- Maintenance costs: High-temperature operations accelerate equipment wear.
- Regulatory compliance: Meeting environmental standards can be costly.
Operating Costs of Pyrolysis
Pyrolysis operating costs are influenced by:
- Feedstock costs: Often lower as waste materials can be used.
- Technology costs: Advanced reactors and control systems can be expensive.
- Emission control systems: Necessary to meet environmental regulations.
Market Trends
Demand for Calcination Processes
The demand for calcination is driven by industries such as:
- Construction: for cement and lime.
- Metal processing: for ore treatment and refining.
Demand for Pyrolysis Processes
Increasing interest in sustainable and waste-to-energy solutions boosts the demand for pyrolysis. Key drivers include:
- Renewable energy production: using biomass to produce biofuels.
- Waste reduction: converting plastic and organic waste into valuable products.
Advantages and Limitations
Benefits of Calcination
Industrial Benefits
Calcination helps produce essential materials with improved properties, such as:
- Increased material reactivity.
- Enhanced purity of metals and minerals.
Environmental Advantages
When powered by renewable energy sources, calcination can have reduced carbon footprints.
Benefits of Pyrolysis
Recycling Potential
Pyrolysis supports the circular economy by:
- Converting waste into resources, reducing landfill use.
- Producing recyclable materials like biochar, which can improve soil fertility.
Energy Production
Pyrolysis effectively generates renewable energy from biomass, contributing to energy diversification.
Challenges Faced
Technical Challenges in Calcination
Challenges include:
- High energy requirements.
- Emission control to meet strict environmental standards.
Technical Challenges in Pyrolysis
Pyrolysis faces issues such as:
- Feedstock variability affecting process stability.
- Scaling up technology for large-scale commercial application.
Future Directions
Innovations and Research
Cutting-Edge Research in Calcination
Research focuses on:
- Developing low-temperature calcination processes to save energy.
- Integrating CO2 capture technologies to mitigate environmental impacts.
Emerging Trends in Pyrolysis
Emerging trends include:
- Enhancing the quality of pyrolysis products for broader applications.
- Modular and mobile pyrolysis units that can be used onsite for waste processing.
Sustainable Practices
Enhancements for Eco-Efficiency
Efforts are underway to:
- Optimize process efficiencies to reduce energy use and emissions.
- Develop sustainable feedstock sources like agricultural waste.
Role in Sustainable Development
Both calcination and pyrolysis play critical roles in:
- Supporting waste reduction initiatives.
- Contributing to renewable energy goals.
- Enhancing material reuse and recycling.
FAQs
What is Calcination?
Calcination is a thermal treatment process that subjects materials to temperatures typically below their melting points to induce phase transition, decomposition, or the removal of volatile fractions. It is often used in the production of cement, ceramics, and the purification of metals.
How Does Pyrolysis Work?
Pyrolysis operates by heating organic material in the absence of oxygen, preventing combustion. This process generates three primary products: solid residue (char), liquid (tar), and gas, each with various applications in energy production and material synthesis.
Are Calcination and Pyrolysis Eco-Friendly?
Both processes can be designed to be more environmentally friendly. Calcination, if powered by renewable energy and equipped with emissions control technologies, can significantly reduce its environmental footprint. Pyrolysis can be part of waste-to-energy solutions, converting waste materials into usable energy forms with lower emissions compared to traditional incineration.
What Materials Can Undergo Pyrolysis?
A wide range of organic materials can undergo pyrolysis, including biomass, plastics, and waste materials. The process is particularly valuable for converting waste into biochar, synthetic gas, and bio-oils, which can be used as fuels or chemical feedstocks.
Conclusion
In summarizing the differences between calcination and pyrolysis, it’s clear that each process holds distinct advantages tailored to specific industrial needs. Calcination is essential for producing inorganic materials with specific properties, while pyrolysis offers a pathway to energy recovery and material recycling from organic waste.
The future of these thermal techniques is promising, with ongoing research aimed at enhancing their efficiency and environmental performance. As global industries lean towards sustainability, the optimization of processes like calcination and pyrolysis will be crucial in minimizing environmental impact while maximizing material and energy outputs.

