Buffers play an indispensable role in maintaining the stability of chemical systems, ensuring that pH levels remain constant despite the addition of acids or bases. These mixtures of weak acids and their conjugate bases (or vice versa) are foundational in both natural and artificial environments, from biological systems to industrial processes. Their capacity to prevent sudden shifts in pH is crucial for the survival of many organisms and the efficiency of numerous chemical reactions.
The difference between buffer action and buffer capacity lies at the heart of understanding how buffers operate. Buffer action refers to the ability of a buffer solution to resist changes in pH when small amounts of acid or base are added. In contrast, buffer capacity is a measure of the extent to which a buffer can resist pH change, determined by both the amount and the ratio of acid and base it contains.
Buffers function by reacting with any added acid or base to maintain the pH within a narrow range. This capability ensures that enzymatic reactions, biological processes, and chemical reactions proceed under optimal conditions. The balance between buffer action and buffer capacity determines a buffer’s effectiveness, influencing its application in various scientific and industrial fields.
Buffer Basics
What is a Buffer?
Buffers are critical components in both natural and industrial chemical processes, designed to maintain stable pH levels in solutions, even when acids or bases are introduced. This stability is essential for processes ranging from metabolic pathways in living organisms to chemical reactions in industrial settings.
Definition and Purpose in Chemical Solutions
A buffer is a special solution that resists changes in pH when small amounts of acid or base are added. The purpose of a buffer is to keep the pH of a solution stable, which is crucial for reactions that are sensitive to changes in acidity or alkalinity. This stability supports a wide range of biological and chemical processes.
Components of a Buffer
Acid and Base Components
Buffers consist of a weak acid and its conjugate base, or a weak base and its conjugate acid. These components work together to neutralize added acids or bases.
How They Work Together
The acid component of a buffer can react with any added bases, while the base component can react with added acids. This dual capability allows buffers to maintain the pH of a solution within a narrow range.

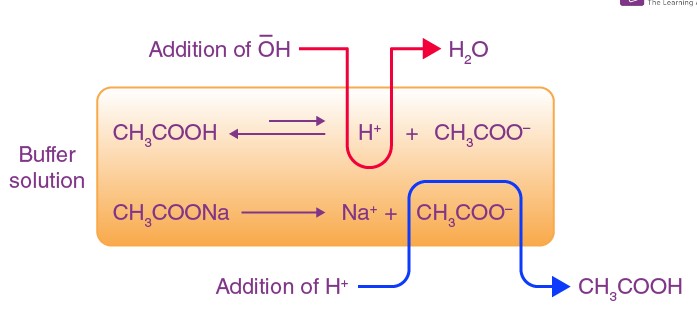
Buffer Action
Buffer Action Explained
Buffer action is the term used to describe how buffers work to maintain pH levels. This action is essential for environments where pH stability is crucial.
Role in Maintaining pH Levels
Buffers play a key role in environments like living cells, where enzymes require specific pH levels to function optimally. By resisting changes in pH, buffers allow these biological processes to continue uninterrupted.
Mechanism of Buffer Action
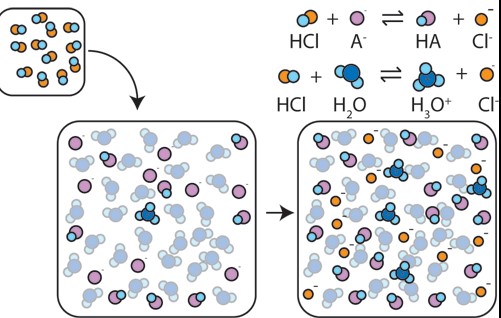
The mechanism of buffer action involves the chemical equilibrium between the weak acid and its conjugate base (or the weak base and its conjugate acid). When an acid or base is added to the solution, the equilibrium shifts to neutralize the added substance without significantly changing the solution’s pH.
Factors Affecting Buffer Action
Concentration of Components
The effectiveness of buffer action depends on the concentration of its acid and base components. Higher concentrations generally lead to stronger buffer action.
pH Range of the Buffer Solution
Each buffer has an optimal pH range where its buffering capacity is strongest. This range is typically near the pKa of the weak acid or base in the buffer.
Buffer Capacity
Defining Buffer Capacity
Buffer capacity is a measure of a buffer’s ability to resist pH change. It indicates how much acid or base the buffer can neutralize before its pH begins to change significantly.
Measuring Buffer Capacity
The buffer capacity is determined by the amount of acid or base needed to change the pH of a buffer solution by one unit. It’s a quantitative measure of a buffer’s performance.
Factors Influencing Buffer Capacity
Ratio of Acid to Base Components
The ratio of acid to base components in a buffer affects its capacity. An optimal ratio can maximize the buffer’s capacity to resist pH changes.
Total Concentration of the Buffer
The total concentration of the buffer components also plays a critical role. A higher total concentration can enhance the buffer’s capacity to maintain pH stability.
Buffer Action vs. Buffer Capacity
Key Differences
When discussing buffer solutions, two critical concepts often come into play: buffer action and buffer capacity. Though closely related, these terms describe different characteristics of buffers that are crucial for their effective use in various scientific and industrial applications.
Summary of Distinct Aspects
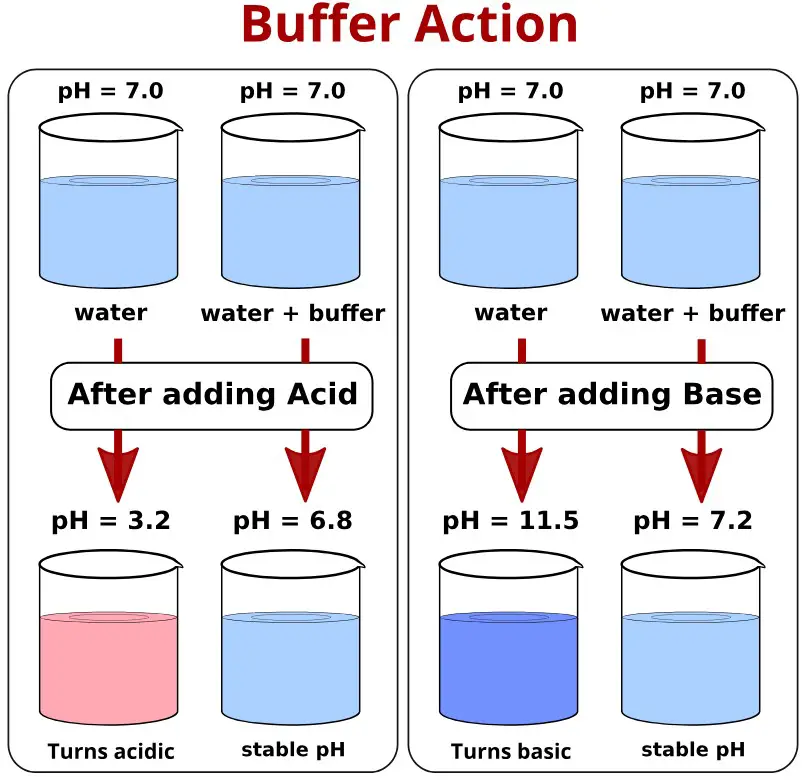
Buffer action refers to a buffer’s ability to maintain a stable pH level upon the addition of small amounts of acids or bases. It is essentially a qualitative measure of a buffer’s effectiveness in resisting changes in pH levels. This action is crucial for processes and reactions that require a consistent pH environment to proceed correctly.
Buffer capacity, on the other hand, quantifies how much acid or base a buffer can neutralize before its pH starts to change significantly. It’s a measure of the buffer’s strength or resilience against pH fluctuations, indicating the volume of acid or base that can be added without causing a substantial shift in pH.
The key difference between these concepts lies in their focus: buffer action is about the ability to resist pH change, while buffer capacity is about the extent to which pH can be maintained before a change occurs.
Practical Implications
The distinction between buffer action and capacity has significant practical implications, especially in biological systems and industrial processes where precise pH control is essential.
Importance in Various Applications
- Biological Systems: In biology, enzymes and proteins often require specific pH levels to function optimally. Buffer solutions are used to maintain these conditions, ensuring that metabolic and biochemical processes proceed without disruption.
- Industrial Processes: Many industrial processes, such as fermentation, drug manufacturing, and chemical production, rely on buffers to control the pH level, affecting the quality, yield, and safety of the final products.
Optimizing Buffers
Selecting and optimizing buffers is a critical step in the design of experiments and industrial processes. The goal is to choose a buffer that provides the desired pH stability while meeting specific application requirements.
Choosing the Right Buffer
The selection of an appropriate buffer depends on several factors, including the pH range for the process, the buffer’s pKa, and the ionic strength needed. Considerations include:
- pH Range: The chosen buffer should have a pKa value close to the desired pH level of the solution to maximize its effectiveness.
- Ionic Strength: The ionic strength of the buffer solution can affect the solubility of compounds and the stability of proteins in biological applications.
- Compatibility: The buffer components should be compatible with other substances in the solution to avoid unwanted chemical reactions.
Adjusting Buffer Capacity
Once the right buffer is chosen, adjusting its capacity to suit specific needs can enhance performance. Methods include:
- Adjusting Concentrations: Increasing the total concentration of buffer components can enhance buffer capacity, allowing the solution to neutralize more added acid or base without a significant pH change.
- Optimizing Component Ratio: Altering the ratio of acid to base components can optimize the buffer’s capacity for specific pH ranges, improving its effectiveness in maintaining pH stability.
Frequently Asked Questions
What is buffer action?
Buffer action is the mechanism by which buffers maintain a stable pH level within a solution, despite the addition of small amounts of acids or bases. This action relies on the chemical equilibrium between the weak acid (or base) and its conjugate base (or acid) present in the buffer solution, allowing it to neutralize added substances and prevent significant pH changes.
How does buffer capacity vary?
Buffer capacity varies based on the concentration of the acid and base components within the buffer solution and their ratio. A higher total concentration and an optimal ratio of acid to base enhance the buffer’s capacity to resist pH changes, making it more effective in stabilizing the pH of a solution over a wider range of added acidic or basic substances.
Why is understanding buffers important?
Understanding buffers is crucial for fields such as biochemistry, environmental science, and pharmaceuticals, where pH stability can impact biological functions, chemical reactions, and the effectiveness of drugs. Buffers ensure that conditions remain optimal for enzymatic activity, metabolic processes, and chemical reactions, highlighting their significance in scientific research and industrial applications.
Conclusion
The distinction between buffer action and buffer capacity is fundamental to the study and application of chemistry. Recognizing this difference not only enhances our comprehension of chemical equilibrium but also informs our selection and optimization of buffers for various purposes. Whether in regulating the pH of blood in the human body or controlling the conditions of a chemical reaction, the principles of buffer action and capacity are key to achieving stability and efficiency.
As we navigate the complexities of chemical reactions and biological processes, the strategic use of buffers becomes a testament to the elegance of chemistry in balancing the delicate interplay of acids and bases. The knowledge of buffer action and capacity empowers scientists and industry professionals to fine-tune conditions for optimal outcomes, underscoring the importance of these concepts in advancing scientific understanding and technological progress.

