In the intricate dance of atoms and molecules that forms the basis of chemistry, two key concepts often emerge as critical to understanding how substances interact and transform: bond enthalpy and lattice enthalpy. These terms, while seemingly esoteric, play pivotal roles in dictating the energy dynamics of chemical bonds—the invisible links that tie atoms together into molecules and compounds.
Bond enthalpy and lattice enthalpy are measures of the energy involved in breaking and forming chemical bonds. Bond enthalpy refers to the energy required to break a bond between two atoms in a molecule, while lattice enthalpy describes the energy released when ions in a gaseous state form an ionic lattice, or the energy required to break apart the lattice into its constituent ions.
These concepts not only shed light on the stability and reactivity of compounds but also have practical implications in fields ranging from materials science to pharmacology. By understanding the energy changes associated with forming and breaking bonds, chemists can predict reaction outcomes, design new materials, and even tailor drugs to interact precisely with biological molecules.
Bond Enthalpy
Basics of Bond Enthalpy
Definition and Importance
Bond enthalpy is the energy required to break a single bond between two atoms in a molecule, with all substances in the gas phase. This concept is foundational in chemical bonding because it quantifies the strength of chemical bonds. High bond enthalpy means a bond is strong and requires more energy to break. This is crucial for predicting the stability of molecules and their reactivity in chemical reactions.
Role in Chemical Bonding
Bond enthalpy affects how molecules interact, react, and change. It helps scientists understand reaction mechanisms, energy changes, and the balance between reactants and products in chemical equations. This knowledge is applied in designing new materials, pharmaceuticals, and in energy production.
Measuring Bond Enthalpy
Measurement Techniques
Bond enthalpy is measured through experimental and theoretical methods, involving calorimetry and computational chemistry, respectively. The process includes:
- Heating substances to initiate reactions.
- Measuring the heat change.
- Calculating the bond enthalpy using the energy absorbed or released.
Influencing Factors
Several factors affect bond enthalpy measurements:
- Temperature: Higher temperatures can alter the energy states of molecules.
- State of Matter: Gaseous state is standard for measurement because it simplifies calculations.
- Bond Type: Single, double, and triple bonds have different enthalpies.
Factors Influencing Bond Enthalpy
Atomic Size and Electronegativity
- Atomic Size: Larger atoms have longer bonds, generally weaker and with lower bond enthalpy.
- Electronegativity: Differences in electronegativity between two atoms affect bond strength. Greater differences usually result in stronger bonds and higher bond enthalpies.
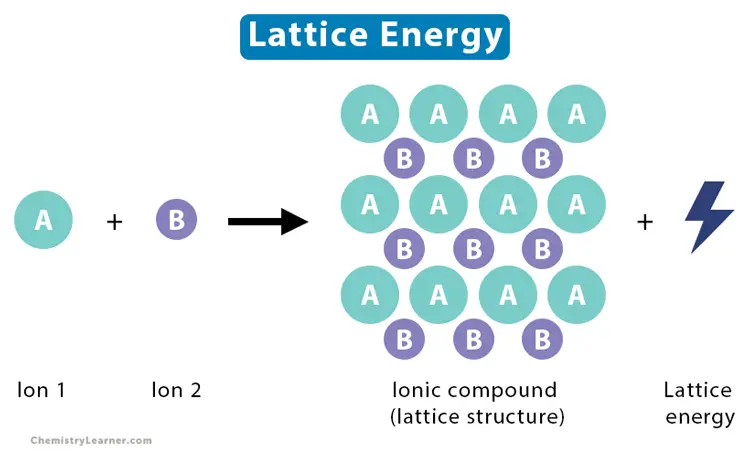
Lattice Enthalpy
Lattice Enthalpy Explained
Definition and Relevance
Lattice enthalpy is the energy released when ions in the gaseous state form an ionic lattice or the energy needed to break an ionic compound into its ions in the gas phase. It’s a key concept for understanding the stability of ionic compounds and their melting and boiling points. High lattice enthalpy indicates a compound is more stable and has higher melting and boiling points.
Importance in Ionic Compounds
This concept is especially relevant for ionic compounds, as it explains why these compounds have high melting and boiling points. Lattice enthalpy is crucial for predicting solubility, designing salts and minerals, and in the field of electrochemistry.
Calculating Lattice Enthalpy
Calculation Methods
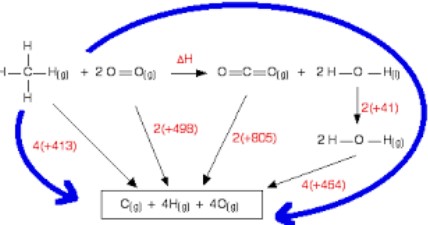
Lattice enthalpy is calculated indirectly using the Born-Haber cycle, a thermodynamic cycle that includes:
- Formation energy of gaseous ions from the elements.
- Ionization energy of the metal.
- Electron affinity of the non-metal.
- Sublimation energy of the solid metal to gas.
- Lattice enthalpy calculated by balancing the cycle.
Born-Haber Cycle
The Born-Haber cycle provides a step-by-step visualization of the energy changes occurring during the formation of an ionic compound. It bridges experimental data with theoretical calculations to estimate lattice enthalpy accurately.
Factors Affecting Lattice Enthalpy
Ionic Charge and Radius
- Ionic Charge: Higher charges on the ions lead to stronger electrostatic attractions and higher lattice enthalpies.
- Ionic Radius: Smaller ions are closer together, increasing the electrostatic force and thus the lattice enthalpy.
Comparison
Key Differences
Bond enthalpy and lattice enthalpy are fundamental concepts in chemistry, each with its unique role and significance.
- Bond Enthalpy: Focuses on the energy needed to break a specific type of bond between two atoms in a molecule. It’s a measure of the bond’s strength and varies with the type of bond (single, double, triple) and the atoms involved. Bond enthalpy is critical for understanding molecular stability, reaction mechanisms, and energy changes in reactions involving covalent compounds.
- Lattice Enthalpy: Relates to ionic compounds, describing the energy released when ions form a solid lattice or the energy required to separate the lattice into gaseous ions. It provides insights into the compound’s stability, melting and boiling points, and solubility. Lattice enthalpy is influenced by the ionic charge and the size of the ions.
The key difference lies in their application: bond enthalpy applies to covalent bonds within molecules, while lattice enthalpy pertains to the ionic bonds in a crystal lattice. These distinctions highlight the varied energy considerations in different types of chemical bonds.
Energy Calculations
Both bond and lattice enthalpy play critical roles in energy calculations, essential for predicting reaction outcomes and understanding energy requirements or releases.
- Bond Enthalpy: Used in calculating the energy change in chemical reactions involving covalent bonds. By summing the bond enthalpies of bonds broken and formed, chemists can estimate the overall energy change of a reaction. This calculation is pivotal for reaction feasibility studies and in designing energy-efficient synthesis pathways.
- Lattice Enthalpy: Integral to calculations involving ionic compounds, especially in solubility, formation, and dissolution processes. The lattice enthalpy helps in understanding the energy landscape of forming an ionic solid from its constituent ions or vice versa. This understanding is crucial in processes like salt formation, mineral extraction, and in predicting the behavior of ionic substances in different environments.
Relevance to Chemical Stability
The stability of chemical compounds is greatly influenced by both bond and lattice enthalpy.
- Bond Enthalpy contributes to molecular stability; molecules with high bond enthalpies have stronger bonds that are less prone to breaking, making the compounds more stable. This stability is crucial in determining the reactivity of a compound, its lifespan, and its suitability for various applications, from pharmaceuticals to materials science.
- Lattice Enthalpy is a determinant of the stability of ionic compounds. High lattice enthalpies indicate strong electrostatic forces between ions, resulting in higher melting and boiling points, and lower solubility in polar solvents. This stability is key in applications where high thermal resistance or low solubility is desired.
Applications
In Thermodynamics
The concepts of bond and lattice enthalpy are integral to thermodynamics, providing insights into the energy changes associated with chemical reactions and phase changes.
- Bond Enthalpy: Helps in understanding the enthalpy changes in reactions, guiding the prediction of reaction spontaneity and the direction of chemical processes. This understanding is crucial in thermodynamic analysis and in the optimization of chemical reactions for industrial processes.
- Lattice Enthalpy: Plays a role in the thermodynamic assessment of melting and boiling points, crystal formation, and dissolution processes. Knowledge of lattice enthalpy aids in predicting phase behavior, which is essential in the design of separation processes and in the manufacturing of crystalline materials.
Industrial Applications
Understanding bond and lattice enthalpy is critical in various industries, impacting the design, synthesis, and utilization of chemical products.
- Pharmaceuticals: Knowledge of bond enthalpy aids in designing drugs with the desired reactivity and stability. Lattice enthalpy is important in understanding the solubility and bioavailability of ionic compounds used as drugs or in drug formulations.
- Materials Science: The stability and reactivity predicted by bond and lattice enthalpies guide the synthesis of new materials, including polymers, ceramics, and metals. This understanding is pivotal in developing materials with specific properties, such as high strength, thermal stability, or electrical conductivity.
Research Implications
The study of bond and lattice enthalpy continues to push the boundaries of research, leading to the development of new compounds and materials with novel properties.
- New Compound Synthesis: Insights into bond and lattice enthalpy enable researchers to predict the stability and reactivity of newly synthesized compounds, guiding the search for substances with specific functions or activities.
- Material Innovation: Understanding the energy involved in forming and breaking bonds aids in designing materials with desired characteristics, such as enhanced durability, improved efficiency in energy storage and conversion devices, and novel pharmaceutical formulations.
Frequently Asked Questions
What is bond enthalpy?
Bond enthalpy, also known as bond energy, is a measure of the strength of a chemical bond. It represents the amount of energy required to break one mole of bonds in a substance, in the gas phase, into individual atoms. High bond enthalpy values indicate strong bonds that are less likely to break, which contributes to the stability of a molecule.
How is lattice enthalpy measured?
Lattice enthalpy cannot be measured directly but is instead calculated from other thermodynamic data, often through the Born-Haber cycle. This cycle involves a series of steps that represent the formation of an ionic compound from its elements, allowing the indirect determination of lattice enthalpy by considering the energies involved in each step.
Why are bond and lattice enthalpy important?
Bond and lattice enthalpy are crucial for understanding the stability of chemical compounds and the energy changes that occur during chemical reactions. Knowledge of these values helps predict reaction pathways, understand phase changes, and design compounds with desired properties for use in various industrial and pharmaceutical applications.
How do factors like atomic size affect bond enthalpy?
Atomic size significantly impacts bond enthalpy. Generally, as the size of the atoms involved in a bond increases, the bond length also increases, leading to a decrease in bond enthalpy. This is because larger atoms have their valence electrons farther from the nucleus, resulting in weaker attractions and thus bonds that require less energy to break.
Conclusion
The exploration of bond enthalpy and lattice enthalpy unravels the intricate energy landscape of chemical interactions, offering deep insights into the forces that drive chemical reactions and the formation of compounds. These concepts serve as fundamental tools for chemists and scientists, enabling them to predict the behavior of molecules, design new materials, and understand the underlying principles of energy transfer in chemistry.
In closing, the distinction between bond enthalpy and lattice enthalpy underscores the complexity of chemical bonds and their pivotal role in the fabric of matter. By appreciating these nuances, one can better comprehend the vast and dynamic world of chemistry that underpins not only the material universe but also the myriad processes that sustain life itself.
