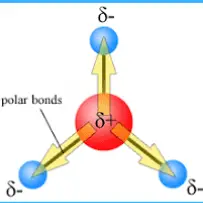
The distinction between bond dipole and molecular dipole moments is fundamental in the study of chemical molecules and their interactions. These concepts play a critical role in predicting the behavior of molecules in various environments and are key to understanding molecular polarity. By examining the distribution of electrical charges in a molecule, scientists can determine how it will interact with its surroundings.
Bond dipole moments arise from differences in electronegativity between two atoms in a bond, leading to a partial positive charge on one end and a partial negative charge on the other. Conversely, a molecular dipole moment is the vector sum of all bond dipoles in a molecule, influenced by the molecule’s shape and symmetry. If the vector sum of all bond dipoles results in a nonzero total, the molecule has a molecular dipole.
Dipole moments influence many physical properties of substances, including boiling points, solubility, and reactivity. For instance, molecules with significant dipole moments tend to have higher boiling points due to strong intermolecular dipole-dipole interactions. Understanding these concepts allows chemists and researchers to predict and manipulate the properties of substances for various applications, enhancing everything from pharmaceuticals to nanotechnology.
Basic Concepts
Dipole Moments
Definition and Significance
A dipole moment is a measure of the separation of positive and negative electrical charges within a molecule. This separation creates a polar molecule with distinct electrical poles. The magnitude of the dipole moment directly relates to the strength of the molecular polarity and influences how molecules interact with electric fields and other molecules. The concept is crucial for predicting the physical properties of substances, such as their boiling points, solubility, and reactivity.
Polar and Non-Polar Molecules
Characteristics and Examples
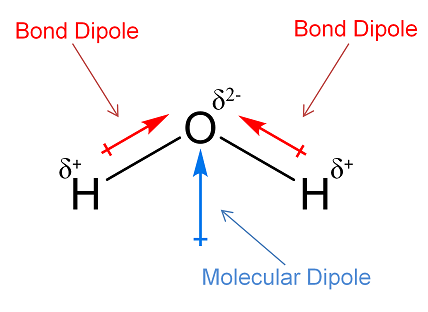
Molecules are classified as polar or non-polar based on their dipole moments. Polar molecules exhibit a significant dipole moment due to unequal electron sharing in chemical bonds and an asymmetrical arrangement of these bonds. Water (H2O) is a classic example, where the oxygen atom holds electrons more tightly than hydrogen, creating a molecule with a distinct positive and negative side.
In contrast, non-polar molecules have no overall dipole moment. This occurs either when atoms share electrons equally, as in the oxygen molecule (O2), or when symmetrical shapes cause bond dipoles to cancel out, as seen in methane (CH4).
Bond Dipole
Definition
A bond dipole is defined by the difference in electronegativity between two bonded atoms, resulting in partial charges at each end of the bond. This concept is key to understanding molecular structure and behavior.
Explanation of Bond Dipole Moments
The bond dipole is represented by a vector pointing from the positively charged atom to the negatively charged atom. The length of the vector indicates the magnitude of the dipole, while the direction shows the polarity of the bond.
Factors Affecting Bond Dipole
Electronegativity Differences
The primary factor influencing bond dipoles is the electronegativity of the atoms involved. Electronegativity refers to an atom’s ability to attract electrons. For example, in a water molecule, oxygen is more electronegative than hydrogen, creating a strong dipole moment.
Bond Length Considerations
The length of the bond also impacts the dipole moment. Longer bonds tend to have more pronounced dipole moments due to the greater separation between charges. This relationship is particularly visible in comparisons between single, double, and triple bonds, with longer single bonds generally exhibiting larger dipoles.
Molecular Dipole
Definition
A molecular dipole refers to the sum of all bond dipoles within a molecule considered as a whole. This sum depends not only on individual bond dipoles but also on the molecule’s geometry.
Explanation of Molecular Dipole Moments
To determine the molecular dipole, one must consider both the magnitude and direction of each bond dipole. The molecular dipole is influenced by how these vectors add together, taking into account the three-dimensional structure of the molecule.
Factors Influencing Molecular Dipole
Shape of Molecule
The shape of the molecule plays a critical role in determining its dipole moment. Molecules with symmetrical shapes, such as carbon dioxide (CO2), may have high bond dipoles that cancel out, resulting in no overall molecular dipole.
Symmetry and Vector Sum
Symmetry determines how bond dipoles add together. For molecules like benzene, symmetry causes the bond dipoles to cancel each other, leading to a non-polar molecule despite polar bonds. The vector sum of bond dipoles, considering both direction and magnitude, determines whether a molecule has a net dipole moment.
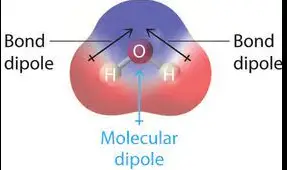
Bond vs. Molecular Dipole
Key Differences
When distinguishing between bond dipoles and molecular dipoles, it’s essential to consider both their origins and impacts. A bond dipole results from the difference in electronegativity between two bonded atoms, causing an uneven distribution of electron density. On the other hand, a molecular dipole is the vector sum of all bond dipoles in a molecule, influenced by the molecule’s overall shape and symmetry.
Detailed Comparison of Factors
Bond dipoles are localized within a molecule, affecting only the atoms they connect. In contrast, the molecular dipole affects the molecule as a whole, influencing how it interacts with other molecules and external fields. The key factors include:
- Electronegativity: More pronounced in bond dipoles.
- Molecular geometry: Primarily influences molecular dipoles, with symmetrical molecules potentially having no net dipole despite having polar bonds.
Examples and Analysis
Case Studies of Common Molecules
Water (H2O): A classic example where two strong bond dipoles due to hydrogen and oxygen result in a significant molecular dipole. Carbon Dioxide (CO2): Despite having two strong bond dipoles, the linear, symmetrical shape of CO2 results in no overall molecular dipole.
Calculation Methods
Measuring Dipole Moments
To measure the dipole moments of molecules, scientists use a variety of techniques and tools:
- Debye Spectroscopy: Measures the orientation of molecular dipoles in an electric field.
- Infrared Spectroscopy: Utilizes the absorption of infrared radiation to infer dipole moments based on molecular vibrations.
Calculating Examples
Step-by-step Computational Guide
- Identify Bond Polarity: Determine which bonds are polar by comparing electronegativity values.
- Determine Bond Angles: Use molecular geometry to find the angles between bond dipoles.
- Calculate Vector Sum: Add bond dipoles vectorially to find the net molecular dipole.
Applications
Importance in Chemistry
Understanding dipole moments is vital for predicting the chemical properties and reactions of substances. Polar molecules, due to their dipoles, can engage in hydrogen bonding and other dipole-dipole interactions, which are crucial for processes like solvent interactions and biochemical reactions.
Real-World Uses
Industrial and Research Implications
In the pharmaceutical industry, dipole moments guide the design of drugs to improve solubility and efficacy. In materials science, engineers use knowledge of dipole moments to create advanced polymers and nanomaterials with specific properties, such as enhanced conductivity or reactivity.
FAQs
What is a bond dipole?
A bond dipole is the difference in electrical charge distribution between two atoms in a chemical bond, due to a difference in their electronegativities. This results in one end of the bond having a partial positive charge and the other a partial negative charge, creating a dipole.
How is a molecular dipole different from a bond dipole?
A molecular dipole is the overall dipole moment of a molecule, calculated as the vector sum of all individual bond dipoles. It is influenced by the molecule’s geometry and the orientation of its bonds, whereas a bond dipole is specific to the charge difference across a single bond.
Why are dipole moments important?
Dipole moments are crucial for understanding the physical properties of molecules, such as boiling points, solubility, and their behavior in electric fields. They help predict how molecules will interact with each other and with solvents, impacting everything from material science to biochemistry.
Can a molecule have bond dipoles but no molecular dipole?
Yes, a molecule can have individual bond dipoles but no overall molecular dipole if the bond dipoles cancel each other out due to the molecule’s symmetry. This is common in molecules like carbon dioxide and boron trifluoride.
Conclusion
The concepts of bond dipole and molecular dipole are essential in the realm of chemistry, providing deep insights into the behavior of molecules based on their polarity. Their study not only aids in predicting the physical and chemical properties of substances but also plays a crucial role in the development of new materials and technologies.
By understanding these dipoles, scientists and engineers can tailor materials with desired properties for specific applications, making the study of dipole moments a cornerstone in the advancement of chemical sciences. The exploration of these fundamental concepts continues to open new avenues in research and industrial applications, highlighting the ongoing importance of chemistry in solving real-world problems.