The exploration of atomic models marks a pivotal chapter in the annals of physics, spotlighting the relentless human quest to unravel the mysteries of the atom. From the earliest notions of indivisible particles to sophisticated quantum mechanical models, our understanding of atomic structure has undergone profound evolution. Central to this journey are the models proposed by Ernest Rutherford and Niels Bohr, each a cornerstone in the edifice of modern physics.
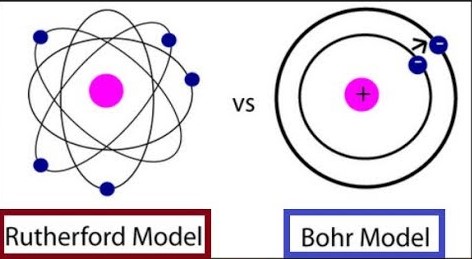
The difference between the Bohr and Rutherford models lies primarily in their depiction of electron behavior and atomic structure. Rutherford’s model introduced the concept of a nuclear atom with electrons orbiting a central nucleus, akin to planets around the sun. Conversely, Bohr’s model advanced this theory by introducing quantized electron orbits, where electrons could only inhabit certain allowed orbits, thus preventing the atom from collapsing due to electromagnetic radiation.
While Rutherford’s model laid the groundwork by identifying the nucleus, it was Bohr’s quantum leap that resolved the puzzle of atomic stability and electron dynamics. This insight into quantized orbits not only explained atomic emission spectra but also paved the way for quantum mechanics, a branch of physics that continues to dazzle with its predictions and applications.
Early Atomic Theories
Brief History Leading to Rutherford’s Model
The quest to understand the atom began with Democritus, who first suggested the existence of indivisible particles, which he called atoms. This idea lay dormant until the 19th century when John Dalton proposed that each element consists of atoms of a single, unique type. Dalton’s atomic theory provided a framework for understanding chemical reactions in terms of atoms recombining in different ways.
As science progressed, so did the complexity of atomic theory. J.J. Thomson discovered the electron, showcasing that atoms were divisible, thus contradicting Dalton’s theory. Thomson proposed the plum pudding model, which depicted the atom as a positive sphere with electrons embedded within it, akin to raisins in a pudding.
Rutherford’s Gold Foil Experiment
Ernest Rutherford, building on Thomson’s work, conducted what is now famously known as the gold foil experiment. Rutherford and his team fired alpha particles at a thin sheet of gold foil. Most particles passed through, but some were deflected at large angles, and a few even bounced back. This unexpected result led Rutherford to conclude that the atom must have a small, dense, positively charged nucleus. This nucleus accounted for only a tiny part of the atom’s volume but contained most of its mass.
Limitations of Previous Models
Thomson’s plum pudding model could not explain the gold foil experiment results. If the positive charge was spread out evenly, as his model suggested, the alpha particles would not have been deflected so dramatically. This experiment highlighted the need for a new model that could account for these findings.
Rutherford’s Model
Core Concepts and Discoveries
Rutherford’s model introduced the concept of a nuclear atom, where electrons orbit a central nucleus, similar to planets orbiting the sun. This was a significant shift from the idea of an atom as a uniform blob of positive charge.
The Nuclear Atom
The nuclear atom model posited that the nucleus is dense, positively charged, and contains most of the atom’s mass. The electrons, being negatively charged, orbit this nucleus at considerable distances, resulting in an atom that is mostly empty space.
Model’s Strengths and Weaknesses
Strengths:
- Explained the results of the gold foil experiment.
- Introduced the nucleus concept, which was a pivotal discovery.
Weaknesses:
- Could not explain why electrons did not spiral into the nucleus as their circular motion should lead to energy loss through radiation.
- Lacked an explanation for atomic spectra.
Bohr’s Model
Introduction to Niels Bohr’s Contributions
Niels Bohr, a Danish physicist, proposed a model that built on Rutherford’s findings while addressing its shortcomings. Bohr had studied under Rutherford and was well-versed in the problems of the nuclear atom model.
Postulates of Bohr’s Model
Bohr introduced several revolutionary ideas, including:
- Electrons orbit the nucleus in fixed orbits or shells without losing energy.
- These orbits represent quantized energy levels. Electrons can move between these levels by absorbing or emitting energy, but they cannot exist between them.
- The energy absorbed or emitted is related to the difference in energy levels, explaining atomic spectra.
How it Addressed Rutherford’s Model Limitations
Bohr’s model provided a solution to the problem of electron collapse and introduced the concept of quantized energy levels, which explained the observed spectral lines of atoms.
Key Differences
Core Structure
Rutherford’s nucleus vs. Bohr’s orbits: Rutherford introduced the concept of a central nucleus, while Bohr expanded on this by defining specific orbits for the electrons.
Energy Levels
Absence in Rutherford, presence in Bohr: Bohr’s model introduced quantized energy levels for electrons, a concept absent in Rutherford’s model.
Electron Behavior
Classical vs. quantum physics approach: Rutherford’s model was rooted in classical physics, unable to explain atomic stability. Bohr’s model used quantum principles to describe electron behavior, paving the way for modern quantum mechanics.
Experimental Support
Both models were tested through various experiments, but Bohr’s model gained more support due to its ability to explain phenomena such as atomic spectra, which Rutherford’s could not.
Scientific Impact
On Atomic Theory
The Bohr and Rutherford models significantly advanced our understanding of atomic structure. They laid the groundwork for the development of quantum mechanics, a theory that has fundamentally changed our view of particles at the microscopic level. Before these models, atoms were thought to be indivisible or merely a cloud of charge. Rutherford’s discovery of the nucleus and Bohr’s introduction of quantized orbits were pivotal, illustrating that atoms have a complex internal structure.
These models helped scientists realize that electrons orbit the nucleus in specific layers or shells and that these electrons can jump between orbits by emitting or absorbing energy. This was a major step forward in explaining chemical properties and reactivities of elements based on their atomic structure, leading to the development of the periodic table in a way that reflects the electronic structure of atoms.
Technological Advancements
The implications of Bohr and Rutherford’s work extended far beyond academic theory; they have had a profound impact on modern technology and research. For instance, the concept of quantum jumps led to the invention of the laser, a device that relies on stimulating electrons to move to higher energy levels and then emit light as they return to lower levels.
Additionally, understanding atomic structure has been crucial in developing various medical imaging techniques, such as MRI and PET scans, which rely on principles of nuclear physics. On a more fundamental level, the semiconductor industry, which is the backbone of all modern electronics, could not exist without a detailed understanding of atomic and quantum physics. Semiconductor devices, like transistors, operate on principles that directly arise from quantum mechanics and the understanding of electron behavior in different materials.
Model Limitations
Rutherford’s Model
While groundbreaking, Rutherford’s model had its limitations. It could not explain why negatively charged electrons in motion around a positively charged nucleus would not lose energy and spiral inward, leading to the collapse of the atom. This was a significant inconsistency that questioned the stability of atoms. Moreover, it failed to account for the observed spectra of elements, indicating that while it was a step in the right direction, it was not the complete picture.
Bohr’s Model
Bohr’s model addressed some of the shortcomings of Rutherford’s by introducing quantized orbits. However, it, too, had limitations. It worked well for hydrogen, the simplest atom, but struggled to accurately predict spectra for more complex atoms. Additionally, Bohr’s model was based on arbitrary rules about electron orbits that had no theoretical foundation at the time, making it a somewhat ad hoc solution.
Beyond Bohr and Rutherford
Introduction to Quantum Mechanics
The limitations of Bohr’s and Rutherford’s models set the stage for the development of quantum mechanics, a branch of physics that studies the behavior of energy and matter at the smallest scales. Quantum mechanics provides a comprehensive framework for understanding the behavior of particles at the atomic and subatomic levels, incorporating the wave-particle duality of matter and energy, the uncertainty principle, and the concept of superposition.
This theory has been successful in explaining phenomena that classical physics could not, such as the structure of complex atoms, the behavior of electrons in materials, and the nature of chemical bonds. Quantum mechanics has not only validated the foundational aspects of Bohr’s model but also expanded upon them, offering a more detailed and accurate description of atomic behavior.
Current Models of the Atom
Today, the standard model of particle physics offers the most accurate and comprehensive description of the atom. This model includes a host of subatomic particles beyond electrons, protons, and neutrons, such as quarks and leptons, and forces like the strong nuclear force that holds the nucleus together. The standard model also incorporates quantum mechanics and special relativity, providing a unified description of the three fundamental forces of nature: electromagnetic, weak, and strong nuclear forces.
Quantum field theory, a key component of the standard model, describes particles as excitations in fields that permeate space and time. This approach has led to predictions and discoveries of particles like the Higgs boson, validating the model’s accuracy. However, the journey of understanding the atom is far from over. Physicists continue to search for a theory of quantum gravity and investigate the dark matter and dark energy that make up most of the universe, challenges that remind us of the continuing evolution of atomic theory.
Frequently Asked Questions
What did Rutherford’s model lack?
Rutherford’s model, while groundbreaking, lacked an explanation for the stability of atoms. It proposed electrons orbiting a nucleus but couldn’t justify why these moving electrons didn’t lose energy and collapse into the nucleus, a question that remained unanswered until the advent of Bohr’s model.
How did Bohr’s model improve upon Rutherford’s?
Bohr’s model introduced quantized electron orbits, suggesting that electrons could only occupy certain defined orbits and would not radiate energy as long as they remained in these orbits. This concept of quantization was revolutionary, addressing the stability issue in Rutherford’s model and laying foundational stones for quantum mechanics.
Why is quantum mechanics important in understanding atoms?
Quantum mechanics is crucial for understanding atoms because it provides a comprehensive framework that explains the behavior of particles at the atomic and subatomic levels. It goes beyond classical models, offering explanations for phenomena such as electron configurations, chemical bonding, and the properties of materials at the quantum level.
Conclusion
The journey from Rutherford’s nuclear atom model to Bohr’s quantum model illustrates a fundamental transition in our understanding of the atom, from classical physics to the quantum realm. These models underscore the iterative nature of scientific discovery, where each new theory builds upon and refines its predecessors, pushing the boundaries of our knowledge and technological capabilities.
Today, the legacy of both Rutherford and Bohr continues to inspire physicists and researchers, reminding us of the power of theoretical models to not only explain the natural world but also to forecast new phenomena and guide the development of novel technologies. As we venture further into the quantum age, the contributions of these pioneering scientists remain invaluable, marking waypoints in humanity’s ongoing quest to decode the universe’s most intricate mysteries.