Chemical bonds form the backbone of molecular structure, dictating the physical and chemical properties of substances. Among the various types of bonds, back bonding and coordinate bonding stand out for their unique roles in the formation of complex structures. These bonding types not only enrich our understanding of chemical interactions but also play pivotal roles in numerous applications, from industrial processes to biological systems.
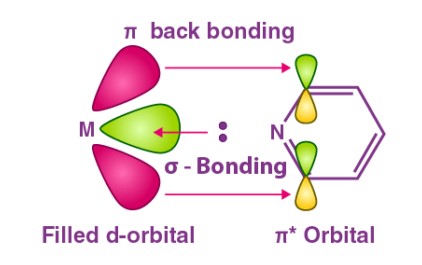
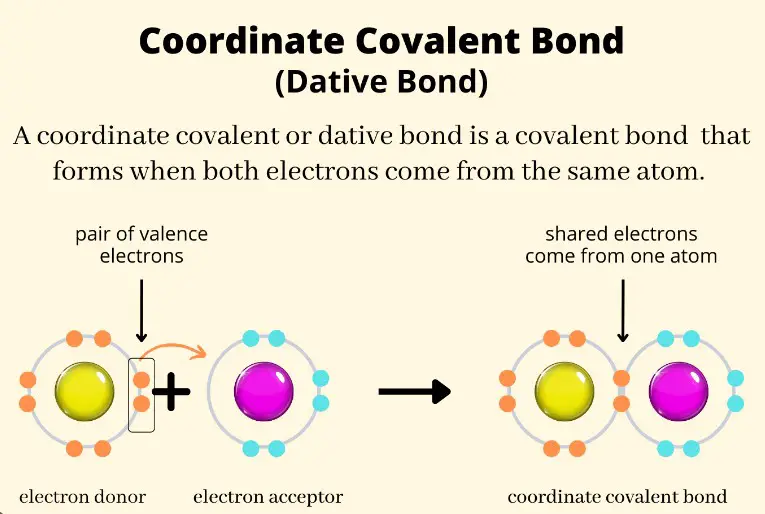
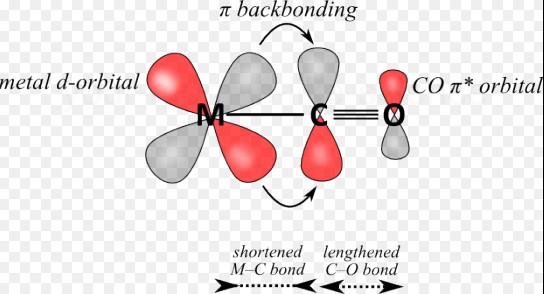
Back bonding is a type of chemical bond where a filled orbital of one atom donates an electron pair to an empty or partially filled orbital of another atom, typically involving π-orbitals. Meanwhile, coordinate bonding, also known as dative covalent bonding, occurs when one atom donates a pair of electrons to another atom without any reciprocal donation. These mechanisms highlight the diversity and complexity of atomic interactions within molecules.
The distinction between back bonding and coordinate bonding lies in their formation process, the nature of electron sharing, and the elements involved. While both play critical roles in chemical structure and reactivity, their differences underline the intricate nature of chemical bonds. Understanding these distinctions is essential for grasping the nuanced behaviors of compounds and their applications in various fields.
Basic Concepts
Chemical Bonds: Definition and Significance
Chemical bonds are the forces that hold atoms together in molecules. They form when atoms share or transfer electrons, creating stability in the structure of the molecule. These bonds are essential because they determine the physical and chemical properties of substances, such as boiling points, melting points, and reactivity. There are several types of chemical bonds, including ionic, covalent, metallic, back bonding, and coordinate bonding, each playing a unique role in the structure and function of molecules.
Back Bonding: Basic Definition
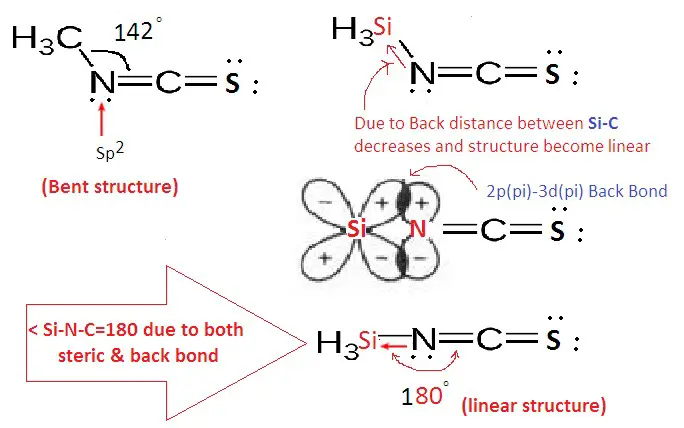
Back bonding is a special type of chemical bond where electron pairs from filled orbitals of one atom are shared with empty or partially filled orbitals of another atom. This process often involves π-orbitals and is significant in molecules where an atom has empty orbitals capable of accepting electron pairs from a neighboring atom. Back bonding is most commonly observed in transition metals and boron family elements, where it plays a crucial role in stabilizing compounds by distributing electron density.
Coordinate Bonding: Basic Definition
Coordinate bonding, also known as dative covalent bonding, occurs when one atom donates a pair of electrons to form a bond with another atom that has an empty orbital but does not reciprocate the electron donation. This type of bonding is crucial in the formation of complexes where metal ions bind with ligands. The donor atom, usually a nonmetal, possesses a lone pair of electrons that it shares with the acceptor atom, often a metal ion, resulting in a stable complex.
Key Differences
Formation Process
How Back Bonding Forms
Back bonding forms through the interaction of a filled orbital of one atom with an empty or partially filled orbital of another atom. This interaction often involves:
- A donor atom with a surplus of electrons in its outer shell.
- An acceptor atom with available empty orbitals. The electron pair from the donor atom’s filled orbital is shared with the acceptor atom, creating a stable bond that enhances the molecule’s stability.
How Coordinate Bonding Forms
Coordinate bonding forms when an atom with a lone pair of electrons donates those electrons to another atom with an empty orbital. The steps typically involve:
- Identification of a donor atom that has electrons to share.
- Identification of an acceptor atom that can receive electrons. The bond is characterized by the one-sided donation of electron pairs, leading to the formation of a bond where the electrons originate from the same atom.
Bonding Elements
Typical Elements Involved in Back Bonding
Back bonding frequently involves elements such as transition metals and elements of the boron group. These elements are characterized by their ability to participate in π-back bonding, thanks to their vacant d orbitals or p orbitals that can accept electron pairs.
Typical Elements Involved in Coordinate Bonding
In coordinate bonding, nonmetals with lone pairs of electrons, such as nitrogen, oxygen, and sulfur, commonly act as donor atoms. The acceptor atoms are often metal ions or electron-deficient species that can accept electron pairs, leading to the formation of coordinate complexes.
Electron Sharing
Electron Sharing in Back Bonding
In back bonding, electron sharing occurs from the donor atom to the acceptor atom, with the donor atom providing an electron pair from its filled orbital to the acceptor’s empty or partially filled orbital. This sharing results in a bond that can have characteristics of both σ and π bonds, depending on the orbitals involved.
Electron Sharing in Coordinate Bonding
In coordinate bonding, the electron sharing is unidirectional, with the donor atom providing both electrons for the bond. This creates a unique bond where the acceptor atom does not contribute any electrons, distinguishing it from traditional covalent bonds where electron sharing is mutual.
Examples
Back Bonding: Common Examples in Molecules
Back bonding is often observed in compounds like:
- Boranes (Boron-hydrogen compounds), where boron’s empty p orbitals accept electron pairs from hydrogen.
- Transition metal complexes, where metals with vacant d orbitals form back bonds with ligands that have lone pairs.
Coordinate Bonding: Common Examples in Complexes
Examples of coordinate bonding can be found in:
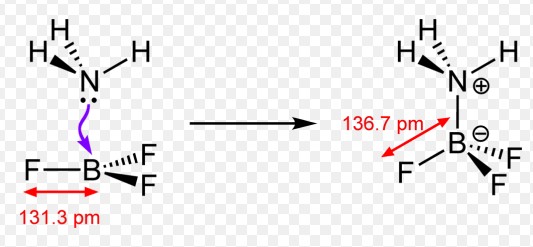
- Ammonia forming a complex with boron trifluoride (BF₃), where nitrogen donates a pair of electrons to boron.
- Metal complexes such as the hexaqua iron (III) ion [��(�2�)6]3+[Fe(H2O)6]3+, where water molecules act as donors to the iron ion.
Bond Characteristics
Strength and Stability
Comparing Bond Strengths
The strength of a chemical bond refers to its ability to withstand external forces without breaking. Back bonding tends to be strong, especially when it involves π-back bonding with transition metals, as these bonds benefit from the overlap between filled d orbitals of the metal and vacant p orbitals of the electron-donor atom. Coordinate bonds are also strong, particularly in metal-ligand complexes, due to the significant electrostatic attraction between the positively charged metal ion and the negatively charged donor atom.
Stability Considerations
Stability in chemical compounds is influenced by the type and strength of the bonds present. Compounds with back bonding often show enhanced stability because the back bond distributes electron density across the molecule, reducing electron deficiency in certain atoms. Similarly, coordinate complexes are stabilized through the formation of coordinate bonds, which fill the electron vacancies of metal ions, leading to stable structures.
Reactivity
How Each Bond Affects Reactivity
Back bonding can decrease a molecule’s reactivity by filling electron-deficient orbitals, making them less likely to participate in chemical reactions. For instance, boranes, stabilized by back bonding, are less reactive than their electron-deficient counterparts. In contrast, coordinate bonding can either increase or decrease reactivity depending on the nature of the complex formed. For example, the formation of a coordinate bond in an enzyme-substrate complex can significantly increase the substrate’s reactivity by properly orienting it for the reaction.
Physical Properties
Influence on Boiling and Melting Points
The physical properties like boiling and melting points are directly influenced by the type of bonding. Molecules with strong back bonding or coordinate bonding interactions exhibit higher boiling and melting points due to the increased energy required to break these interactions. For example, transition metal complexes with extensive back bonding or coordinate bonding interactions often have higher melting points compared to similar compounds without such bonds.
Applications
In Industrial Processes
Utilization of Back Bonding
Back bonding is crucial in catalysis, particularly in processes involving transition metals. For example, the synthesis of ammonia through the Haber process benefits from the back bonding between nitrogen molecules and the metal catalyst, facilitating the breaking of the strong N≡N triple bond. This interaction enhances the catalyst’s effectiveness and efficiency, making the process economically viable.
Utilization of Coordinate Bonding
Coordinate bonding plays a significant role in the purification and extraction of metals through the formation of coordination complexes. In the extraction of copper, for instance, coordinate bonds form between copper ions and ligands in the leaching process, allowing for the selective separation of copper from ore. This method is essential for producing high-purity copper for electrical wiring and other applications.
In Biological Systems
Role of Coordinate Bonding
In biological systems, coordinate bonding is fundamental to the function of metalloproteins and enzymes. Hemoglobin, a protein in red blood cells, uses coordinate bonding to bind oxygen molecules via iron ions. This bond is critical for oxygen transport and delivery throughout the body. Similarly, many enzymes rely on coordinate bonds to bind metal ions that act as cofactors, essential for the enzyme’s catalytic activity.
Visualization Techniques
Molecular Orbital Theory
MO Theory in Understanding Bonding
Molecular Orbital (MO) Theory provides a comprehensive framework for understanding chemical bonding, including back bonding and coordinate bonding. MO theory describes how atomic orbitals combine to form molecular orbitals, which are occupied by the molecule’s electrons. This theory helps explain the bonding in complexes, predicting their stability, reactivity, and magnetic properties by analyzing the electron configuration in the molecular orbitals.
Spectroscopy and Crystallography
Tools for Observing Back and Coordinate Bonds
Spectroscopy and crystallography are pivotal in observing and analyzing back and coordinate bonds:
- Spectroscopy, including infrared (IR) and nuclear magnetic resonance (NMR), allows scientists to infer the presence of back bonding or coordinate bonding by examining the energy levels and environments of electrons within a molecule.
- Crystallography, especially X-ray crystallography, provides a detailed view of the spatial arrangement of atoms in a crystal, revealing the precise nature of the bonds, including bond lengths and angles, indicative of back bonding or coordinate bonding interactions.
Frequently Asked Questions
What is Back Bonding?
Back bonding occurs when an atom with a filled orbital donates an electron pair to an empty or partially filled orbital of another atom, typically involving π-orbitals. This type of bonding is common among atoms with available d-orbitals and plays a crucial role in stabilizing molecules with electron-deficient atoms.
How Does Coordinate Bonding Differ from Covalent Bonding?
Coordinate bonding, unlike traditional covalent bonding, involves a single atom providing both electrons for the bond formation. The donor atom, having a lone pair, forms a bond with an acceptor atom that has an empty orbital, resulting in a dative covalent bond. This is distinct from covalent bonding, where each atom contributes one electron to the bond.
Why is Understanding Chemical Bonding Important?
Understanding chemical bonding is crucial for predicting the behavior of molecules, their structural properties, and reactivity. Knowledge of different bonding types, including back bonding and coordinate bonding, enables chemists to design and synthesize new compounds with desired properties for various applications, from pharmaceuticals to materials science.
Can Back Bonding and Coordinate Bonding Occur in the Same Molecule?
Yes, back bonding and coordinate bonding can occur within the same molecule, depending on the molecule’s structure and the types of atoms involved. Their coexistence further illustrates the complexity of chemical bonding and the potential for diverse interactions within molecules.
Conclusion
The intricate dance between back bonding and coordinate bonding reveals the complexity and beauty of chemical interactions. These bonds not only define the structural integrity and reactivity of molecules but also pave the way for innovative applications in science and industry. Their differences, rooted in the mechanisms of electron sharing and the elements involved, underscore the diversity of chemical bonds.
Understanding these bonds is more than academic; it’s a window into the microscopic world that shapes our macroscopic reality. As we continue to explore the nuances of chemical bonding, we unlock new possibilities for creating materials and compounds that advance technology, medicine, and environmental sustainability. The study of back bonding and coordinate bonding, therefore, is not just about understanding molecules but also about harnessing their potential to improve the world.