Water is a compound that plays an important role in many chemical reactions. Therefore, it is important to understand the various chemical processes that take place when it is involved. Two of these processes are autoionization and autoprotolysis, which are two different ways that water can be ionized.
Two of these processes are autoionization and autoprotolysis, which are two different ways that water can be ionized. In this blog, we will discuss the difference between autoionization and autoprotolysis and how each process works.
What is autoionization
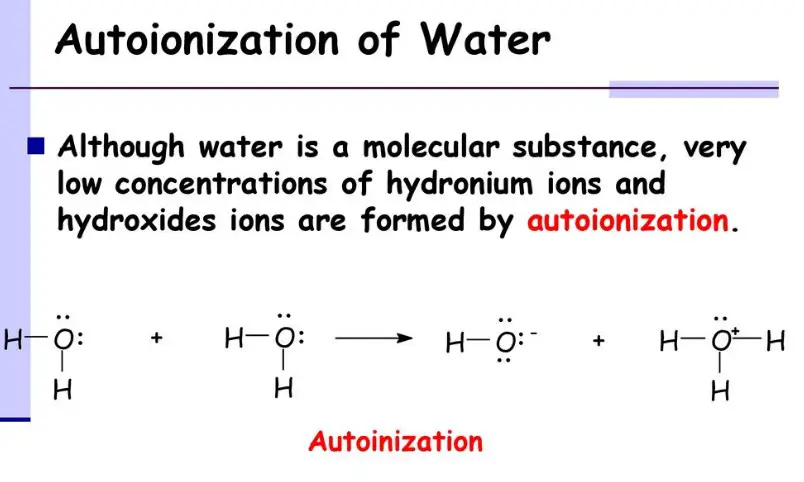
Autoionization is a type of chemical reaction in which a molecule or ion dissociates into two or more smaller molecules or ions. This process occurs spontaneously in aqueous solutions of certain compounds and can be used to produce a variety of products. The difference between autoionization and autoprotolysis is that autoionization involves the transfer of electrons from one molecule or ion to another, while autoprotolysis does not involve electron transfer.
The difference between autoionization and autoprotolysis is that autoionization involves the transfer of electrons from one molecule or ion to another, while autoprotolysis does not involve electron transfer. In autoprotolysis, the molecules or ions simply break apart into smaller pieces. Autoionization is a useful process in many industrial and laboratory applications as it allows for the production of a wide variety of products from a single compound or ion.
What is autoprotolysis

Autoprotolysis is a type of chemical reaction in which a solvent molecule donates a proton to itself. This reaction is the opposite of autoionization, in which a solvent molecule accepts a proton. Autoprotolysis is important in the formation of acid-base equilibria in aqueous solutions.
The difference between autoionization and autoprotolysis is that in autoionization, the solvent molecule acquires a proton whereas in autoprotolysis, the solvent molecule donates a proton to itself. This reaction is important in the formation of acid-base equilibria in aqueous solutions.
Comparison between autoionization and autoprotolysis
When studying the chemical properties of a substance, it’s important to understand the difference between autoionization and autoprotolysis. While both are processes that involve the breakdown of molecules into charged ions, they are distinct processes that have different effects.
Autoionization is the process of a molecule breaking down into two charged ions, while autoprotolysis is the process of a molecule breaking down into two different molecules, with one being a positively charged ion and the other a negatively charged ion. This difference in the resulting product of the reaction is key to understanding the differences between the two processes. Autoionization is a process that occurs in aqueous solutions and involves the breaking of a single molecule into two ions of equal charge, whereas autoprotolysis is a process that occurs in non-aqueous solutions and involves the breaking of a single molecule into two ions of opposite charge.
While both processes involve the breaking down of molecules, the resulting products of the reaction are different and the conditions under which the reaction takes place are also different. Understanding the difference between autoionization and autoprotolysis is key to a comprehensive understanding of chemical properties.
Examples of autoionization and autoprotolysis
Autoionization and autoprotolysis are two processes that involve the transfer of ions within a solution. The primary difference between the two is that autoionization involves the transfer of a single ion, whereas autoprotolysis involves the transfer of two ions. Autoionization is a process in which an ion dissociates from a molecule and forms a new species, while autoprotolysis is a process in which two ions transfer from one molecule to another.
Autoionization is a process in which an ion dissociates from a molecule and forms a new species, while autoprotolysis is a process in which two ions transfer from one molecule to another. Both processes can be used to produce ions from neutral molecules, but the end result of each process is different. Autoionization produces a single ion, while autoprotolysis produces two ions.
Examples of autoionization include the transfer of a proton from a water molecule to form a hydronium ion, and the transfer of a chloride ion from a sodium chloride solution. Examples of autoprotolysis include the transfer of a hydronium ion and a hydroxide ion from a water molecule to form a hydronium hydroxide molecule.
Applications of autoionization and autoprotolysis
Autoionization and autoprotolysis are two similar chemical processes, but there are distinct differences between them. Autoionization is a process in which a molecule or atom spontaneously breaks apart into two or more ions. This process is triggered by the addition of thermal energy to the molecule or atom.
This process is triggered by the addition of thermal energy to the molecule or atom. Autoprotolysis, on the other hand, is a process in which a molecule or atom spontaneously breaks apart into two or more ions due to the presence of an acid or base. In other words, autoionization is a process that is triggered by heat, while autoprotolysis is a process that is triggered by the presence of an acid or base.
Both processes have important applications in a variety of fields, such as medicine, water treatment, and food preservation.
Final Touch
In conclusion, autoionization and autoprotolysis are different processes that involve the dissociation of molecules into ions. Autoionization is the process by which molecules dissociate into positively and negatively charged ions without the involvement of a solvent. Autoprotolysis, on the other hand, is the process by which molecules dissociate into ions with the help of a solvent.
Autoprotolysis, on the other hand, is the process by which molecules dissociate into ions with the help of a solvent. In both processes, the molecules are converted into ions which can be used in various chemical reactions.

