Arsenic and arsine, two substances that evoke caution and concern due to their toxic nature, are often misunderstood and sometimes mistakenly interchanged in discussions. Arsenic, a natural element found in the earth’s crust, has a long history of use in various industries, including agriculture and electronics, despite its poisonous reputation. Arsine, on the other hand, is a gaseous compound that, while less known, poses significant risks in industrial settings.
Arsenic is a semi-metallic element known for its toxicity and widespread presence in the environment, both naturally and from human activities. Arsine, a compound of arsenic, is a colorless, flammable, and highly toxic gas used in some industrial processes. The primary difference between the two lies in their physical state and chemical behavior, with arsenic being solid under standard conditions and arsine existing as a gas.
Arsenic and arsine both have significant health implications and environmental impacts, yet their differences are crucial in understanding how to handle them safely. Arsenic contamination of water and soil can lead to chronic health problems, while arsine exposure is a concern in workplaces involving metals and semiconductors. This distinction underlines the importance of specific precautions and regulations tailored to each substance to mitigate their risks.

Arsenic Overview
Basics of Arsenic
Definition and Characteristics
Arsenic, represented by the chemical symbol As, is a naturally occurring element. With its position in the periodic table under the metalloids category, arsenic displays properties of both metals and non-metals. It’s known for its three allotropic forms: gray, yellow, and black arsenic, with gray being the most stable and common form.
Historical Context and Uses
Historically, arsenic has been used since ancient times for a variety of purposes. Its applications ranged from being a component in bronze alloys to its infamous use as a poison. In the 18th and 19th centuries, arsenic found its way into green pigments for wallpapers and paintings, which inadvertently led to health problems for those in close contact. Today, arsenic is used in the semiconductor industry, in the production of lead-acid batteries, and as a wood preservative.
Health Impacts
Exposure Routes
Exposure to arsenic can occur through ingestion, inhalation, and skin contact. The most common exposure route is through contaminated drinking water, particularly in areas with high natural levels of arsenic. Foods, especially rice and seafood, can also accumulate arsenic, posing dietary risks.
Acute and Chronic Effects
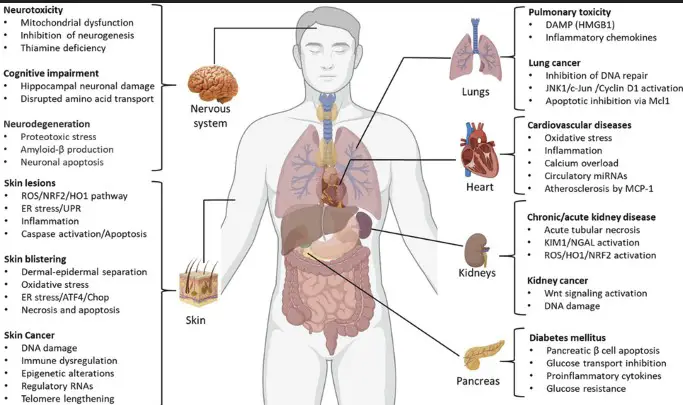
The health effects of arsenic exposure can be acute or chronic. Acute exposure can lead to nausea, vomiting, and even death in severe cases. Chronic exposure, on the other hand, may result in skin lesions, cancer (lung, skin, bladder), cardiovascular diseases, and diabetes.
Environmental Presence
Natural Occurrence
Arsenic is widely distributed in the earth’s crust, typically in conjunction with sulfur and metals. It’s released into the environment through natural processes like volcanic activity and erosion.
Industrial Contributions
Human activities, notably mining, smelting, and the use of arsenic in agriculture and industry, significantly contribute to arsenic contamination of air, water, and soil.
Arsine Overview
Basics of Arsine
Definition and Chemical Properties
Arsine is a gaseous compound of arsenic, with the chemical formula AsH₃. It is colorless, highly toxic, and possesses a garlic-like odor. Due to its extreme toxicity, arsine is considered a chemical hazard in industrial settings.
Industrial Applications
Arsine is primarily used in the semiconductor industry for the synthesis of electronic components. It acts as a dopant in the manufacturing of microchips and integrated circuits.
Health Risks
Exposure Scenarios
Exposure to arsine typically occurs in industrial settings, such as semiconductor manufacturing plants, where the gas is used or produced. Accidental leaks or improper handling can lead to exposure.
Symptoms and Treatment
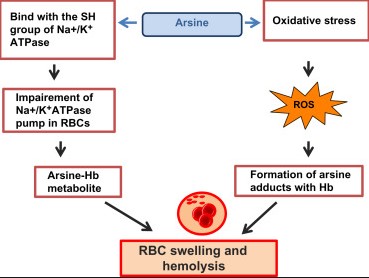
Symptoms of arsine poisoning include headache, dizziness, hemolysis (destruction of red blood cells), and renal failure. Treatment is supportive and focuses on removing the patient from exposure, providing oxygen, and in severe cases, blood transfusions.
Environmental Concerns
Release Mechanisms
Arsine can be released into the environment through industrial emissions and accidental leaks. Its use in electronics manufacturing is a primary source of release.
Mitigation Strategies
To mitigate arsine’s environmental impact, industries employ advanced safety protocols, including gas detection systems, ventilation, and personal protective equipment (PPE) for workers.
Key Differences
Chemical Composition
Elemental vs. Compound
Arsenic is an element found in various forms, whereas arsine is a compound consisting of arsenic and hydrogen.
Structural Distinctions
The key structural difference lies in arsenic being a solid under standard conditions and arsine existing as a gas.
Usage and Applications
Industrial Uses of Arsenic vs. Arsine
While arsenic is used in wood preservation, agriculture, and electronics, arsine finds its primary application in the semiconductor industry as a dopant gas.
Comparative Analysis of Applications
Arsenic’s applications are more varied and extend to traditional uses in agriculture and construction. In contrast, arsine’s use is highly specialized and confined to high-tech industries.
Health and Environmental Impact
Toxicity Levels
Both arsenic and arsine are highly toxic, but their modes of exposure and health effects differ. Arsenic causes problems over long-term exposure, while arsine can have immediate and acute effects.
Exposure Outcomes
The outcome of arsenic exposure is often chronic health issues, including cancer. Arsine exposure, however, can lead to rapid onset of symptoms and potentially fatal outcomes.
Environmental Persistence and Degradation
Arsenic is persistent in the environment, particularly in water and soil. Arsine, being a gas, does not accumulate in the environment but poses risks of acute exposure to humans and wildlife during its release.

Safety and Precautions
Handling Arsenic
Protective Measures
When dealing with arsenic, especially in its powder form, safety is paramount. Workers and individuals should wear protective clothing, including gloves and masks, to prevent skin contact and inhalation. Workplaces must ensure adequate ventilation and use wet methods for handling arsenic to minimize dust.
Detection and Containment
For detection, workplaces should install arsenic-specific monitoring devices that can alert workers to dangerous levels of arsenic in the air. In terms of containment, using closed systems for processing arsenic and employing local exhaust ventilation can significantly reduce the risk of exposure.
Handling Arsine
Safety Protocols
Due to arsine’s extreme toxicity and gaseous state, strict safety protocols are essential. This includes the use of gas-detection systems, self-contained breathing apparatus (SCBA) for workers, and ensuring all handling is done in well-ventilated areas or fume hoods.
Emergency Response
In the event of an arsine leak or exposure, having an emergency response plan in place is critical. This plan should include immediate evacuation, medical treatment for affected individuals, and procedures to safely contain the leak.
Regulatory Perspectives
Arsenic Regulations
Global Standards and Guidelines
Internationally, organizations like the World Health Organization (WHO) provide guidelines for acceptable levels of arsenic in drinking water. The maximum WHO guideline value is 10 µg/L to protect public health.
National Safety Limits
In the United States, the Environmental Protection Agency (EPA) sets the standard for arsenic in drinking water at the same level as WHO, 10 µg/L. Different countries have their own regulations, but most adhere closely to WHO guidelines to ensure public safety.
Arsine Regulations
Industry-specific Regulations
For industries that use or produce arsine, specific regulations mandate the maximum exposure limits. In the US, the Occupational Safety and Health Administration (OSHA) sets permissible exposure limits for arsine gas in the workplace.
Comparison with Arsenic Standards
While arsenic regulations primarily focus on environmental and water safety, arsine regulations are more oriented towards occupational health. This reflects the different nature and risks associated with each substance.
Future Outlook
Technological Advances
Remediation and Safety Technologies
Advancements in technology are continually improving the ways we can detect, handle, and remediate arsenic and arsine. Innovative filtration systems for water and improved personal protective equipment (PPE) are among the developments making environments safer from these substances.
Research Trends
Ongoing research is exploring more efficient and cost-effective methods to remove arsenic from water, soil, and air. Similarly, studies on arsine focus on safer alternatives for industrial processes that currently rely on this hazardous gas.
Policy and Awareness
Evolving Regulations
As scientific understanding of the risks associated with arsenic and arsine grows, regulations are updated to reflect these insights. This includes stricter limits on exposure levels and more comprehensive safety protocols.
Public Awareness Initiatives
Increasing public awareness about the dangers of arsenic and arsine, as well as how to minimize exposure, is crucial. Campaigns focus on educating communities on the risks of contaminated water and promoting safe practices in industries that use these substances.
Implementing Safety Measures for Arsenic and Arsine
The handling and regulation of arsenic and arsine require a multifaceted approach. From the individual to the global level, awareness, education, and strict adherence to safety protocols and regulations are essential to minimize the risks associated with these toxic substances. Whether it’s through personal protective equipment, advanced detection technologies, or community education programs, the goal remains clear: to protect human health and the environment from the harmful effects of arsenic and arsine.
For those in industries dealing with these substances, understanding and implementing the latest safety practices is not just a regulatory requirement but a moral imperative. The same applies to governments and regulatory bodies tasked with protecting public health and worker safety through stringent, science-backed policies.
Frequently Asked Questions
What is Arsenic?
Arsenic is a natural element found in the Earth’s crust, known for its toxicity. It is used in a variety of applications, including pesticides, wood preservatives, and electronic components. Despite its uses, arsenic exposure can lead to serious health issues, including cancer, skin lesions, and cardiovascular diseases.
How is Arsine Produced?
Arsine is produced industrially through chemical reactions involving arsenic compounds and acids. It is primarily used in the semiconductor industry for the synthesis of gallium arsenide, a material used in electronics. Arsine gas is extremely toxic, and its production requires stringent safety measures to prevent accidental exposure.
Can Arsenic be Found in Drinking Water?
Yes, arsenic can be found in drinking water, particularly in areas with high natural levels of arsenic in the groundwater. Long-term exposure to arsenic through contaminated water can lead to serious health problems, including cancer. Water treatment and regular monitoring are essential to reduce arsenic levels in drinking water to safe levels.
What are the Health Effects of Arsine Exposure?
Exposure to arsine gas can lead to immediate health effects, including headache, dizziness, nausea, and in severe cases, hemolysis, leading to kidney failure and death. Due to its high toxicity, arsine exposure requires immediate medical attention and adherence to safety protocols in workplaces.
Conclusion
Understanding the difference between arsenic and arsine is more than an academic distinction; it’s a crucial element in the fields of environmental health and industrial safety. Recognizing the specific properties, uses, and hazards associated with each helps in the implementation of appropriate safety measures and health guidelines to protect individuals and the environment from their toxic effects.
The discourse surrounding arsenic and arsine illustrates the broader challenges and responsibilities in managing toxic substances. It underscores the importance of informed handling, regulatory vigilance, and continuous research to mitigate the risks posed by these and similar compounds. Through diligent practice and policy, we can navigate the complexities they present to public health and safety.

