Organic chemistry, the study of carbon-containing compounds, unfolds a vast array of substances essential to life and industry. Among these, aldehydes stand out due to their unique chemical properties and widespread applications. Their dual nature, categorized into aromatic and aliphatic types, plays a pivotal role in synthesizing a variety of materials and compounds that we encounter daily.
The difference between aromatic and aliphatic aldehydes lies primarily in their structure and the resulting chemical properties. Aromatic aldehydes contain a carbonyl group attached to an aromatic ring, known for their stability and distinct fragrances. On the other hand, aliphatic aldehydes, lacking this ring, often have simpler structures and are known for their reactivity, which makes them invaluable in industrial applications.
Aldehydes, irrespective of their type, serve as foundational blocks in the creation of flavors, fragrances, and synthetic materials. Their study not only enriches our understanding of organic chemistry but also empowers the development of new products and solutions that enhance our quality of life. The exploration of their differences sheds light on their distinct roles and applications, making it a subject of both academic and practical interest.
Aldehydes Basics
Definition and General Structure
Aldehydes are a group of organic compounds characterized by the presence of a carbonyl group (C=O) where at least one hydrogen atom is connected to the carbon atom of the carbonyl group. This structure can be represented as R-CHO, where R can be either a hydrogen atom or an organic substituent, making the molecule either simple or complex. The carbonyl group is key to the reactivity and properties of aldehydes, setting the stage for a variety of chemical reactions.
Role in Chemistry and Industry
Aldehydes play a pivotal role in both chemistry and industry. Their ability to undergo a wide range of chemical reactions makes them essential intermediates in the synthesis of alcohol, acids, plastics, resins, and fragrances. In the food industry, certain aldehydes are used as flavoring agents due to their pleasant scents and tastes. In pharmaceuticals, they serve as building blocks for the synthesis of active pharmaceutical ingredients (APIs). Their widespread applicability underscores the importance of understanding their properties and reactions.
Aromatic Aldehydes
Definition and Structure
Aromatic aldehydes are aldehydes in which the carbonyl group is directly connected to an aromatic ring. This connection significantly influences their chemical behavior and physical properties. The presence of the aromatic ring provides stability to the molecule due to resonance effects, altering the reactivity of the carbonyl group compared to their aliphatic counterparts.
Common Examples
Common examples of aromatic aldehydes include benzaldehyde, known for its almond-like aroma, and vanillin, the primary component of the extract of the vanilla bean. These compounds are widely used in the flavor and fragrance industries, adding desirable aromas and flavors to a variety of products.
Characteristics and Properties
Aromatic aldehydes are characterized by their stability and distinctive aromas. The stability is afforded by the aromatic ring, which delocalizes the electrons of the carbonyl group, making them less reactive than aliphatic aldehydes. Their unique scents make them valuable in perfumery and food flavorings, where they impart complex fragrances and tastes.
Aliphatic Aldehydes
Definition and Structure
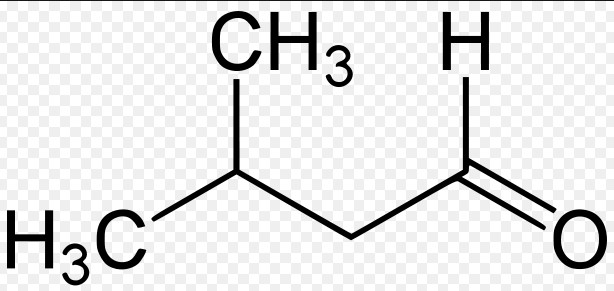
Aliphatic aldehydes lack an aromatic ring and feature a carbonyl group bonded to a straight or branched carbon chain. This structure results in different chemical properties and reactivities compared to aromatic aldehydes. Aliphatic aldehydes can range from simple molecules like formaldehyde to more complex ones like nonanal, which is found in citrus oils.
Common Examples
Formaldehyde is a well-known simple aliphatic aldehyde used in the manufacture of resins and as a disinfectant. Acetaldehyde is another example, involved in the production of acetic acid, perfumes, and flavors.
Characteristics and Properties
Aliphatic aldehydes are generally more reactive than aromatic aldehydes due to the absence of an aromatic ring that stabilizes the carbonyl group. They are known for their sharp and pungent odors. Their reactivity makes them crucial in organic synthesis, where they are involved in numerous reactions including oxidation, reduction, and condensation processes.
Key Differences
Chemical Structure
The main difference in chemical structure between aromatic and aliphatic aldehydes lies in the presence or absence of an aromatic ring. Aromatic aldehydes have a carbonyl group connected to an aromatic ring, while aliphatic aldehydes feature a carbonyl group attached to a non-aromatic carbon chain.
Physical Properties
Aromatic and aliphatic aldehydes differ in boiling points, melting points, and solubility. Aromatic aldehydes generally have higher boiling points due to the presence of the aromatic ring, which contributes to greater van der Waals forces. Aliphatic aldehydes, on the other hand, tend to have lower boiling points and can be more volatile.
Reactivity and Chemical Behavior
Aromatic aldehydes are less reactive towards nucleophilic addition reactions due to the stability imparted by the aromatic ring. Aliphatic aldehydes, lacking this stabilization, are more prone to react with nucleophiles, making them more versatile in synthetic chemistry.
Applications and Uses
The applications of aromatic and aliphatic aldehydes diverge based on their properties. Aromatic aldehydes are extensively used in the fragrance and flavor industries due to their pleasant scents, while aliphatic aldehydes find applications in chemical manufacturing, such as the synthesis of plastics, resins, and organic compounds. This distinction underscores the importance of selecting the appropriate type of aldehyde for specific industrial or synthetic needs.
Chemical Structure and Stability
Bonding and Stability
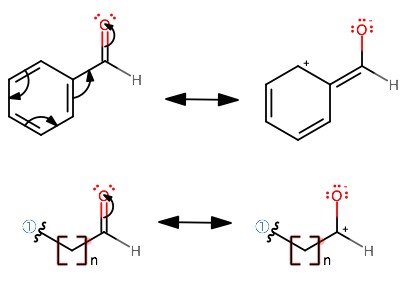
The stability of aldehydes is significantly influenced by their molecular structure and bonding. The carbonyl group, with its double bond between carbon and oxygen, is central to this stability, creating a polarized molecule that can engage in various chemical reactions. Electron delocalization in aromatic aldehydes further enhances stability, as the conjugation with the aromatic ring distributes charge across the molecule, reducing reactivity.
Influence of Aromaticity
Aromaticity adds a layer of stability to aldehydes through resonance stabilization. This phenomenon occurs when the electrons in the carbonyl group are delocalized into the aromatic ring, creating a more stable molecular structure. Aromatic aldehydes, therefore, tend to be less reactive than their aliphatic counterparts, making them more resistant to chemical changes and degradation.
Structural Variations and Their Impact
The impact of structural variations between aliphatic and aromatic aldehydes is profound. The presence of an aromatic ring in aromatic aldehydes not only affects their chemical reactivity but also their physical properties, such as boiling point and solubility. Structural differences also influence the types of reactions these compounds can undergo, with aliphatic aldehydes typically participating in a wider range of chemical transformations.
Physical Properties
Boiling Points and Melting Points
The boiling and melting points of aldehydes are directly influenced by their molecular weight and structure. Aromatic aldehydes generally have higher boiling and melting points due to the pi-stacking interactions of the aromatic rings, which require more energy to break. Aliphatic aldehydes, with their simpler structures, tend to have lower boiling and melting points.
Solubility and Odor
Solubility in water decreases as the length of the aliphatic chain increases, due to the hydrophobic nature of the carbon chain. Aromatic aldehydes, with their polar carbonyl group and nonpolar aromatic ring, exhibit varied solubility depending on the specific compound and its functional groups. The odor of aldehydes is a notable feature, with aromatic aldehydes often having pleasant scents and aliphatic aldehydes presenting a wider range of odors, from pungent to fruity.
Appearance and State
Aldehydes can vary in appearance from colorless liquids to white solids, depending on their structure and molecular weight. Lower molecular weight aldehydes are typically liquids at room temperature, while higher molecular weight compounds may be solids. The presence of an aromatic ring can also influence the state, with some aromatic aldehydes being solid due to additional molecular interactions.
Reactivity and Chemical Behavior
Reaction Mechanisms
Aldehydes undergo a variety of reaction mechanisms, including addition, oxidation, and reduction reactions. The carbonyl group’s polarization makes it an electrophilic site, susceptible to attack by nucleophiles. Aromatic aldehydes, while still reactive, exhibit a reduced rate of reaction due to the stability provided by the aromatic ring.
Susceptibility to Nucleophilic Attacks
The susceptibility of aldehydes to nucleophilic attacks is a key aspect of their reactivity. Aliphatic aldehydes, lacking the stabilization from an aromatic ring, are more prone to these attacks, leading to a broader range of potential reactions and products. This makes them particularly useful in organic synthesis, where they can be transformed into various compounds.
Formation of Products
The products formed from aldehydes depend on the type of reaction they undergo. Common products include alcohols, acids, and esters, each of which has significant industrial and pharmaceutical applications. The specific products can vary widely between aromatic and aliphatic aldehydes due to differences in their reactivity and the types of reactions they can participate in.
Applications and Uses
Aromatic Aldehydes in Perfumery and Flavoring
Aromatic aldehydes are renowned for their use in the perfumery and flavoring industries. Their complex, often pleasant scents make them ideal components of fragrances and flavorings. Benzaldehyde, with its almond scent, and vanillin, known for its warm, vanilla aroma, are prime examples of how aromatic aldehydes enrich consumer products.
Aliphatic Aldehydes in Industrial Applications
Aliphatic aldehydes find extensive applications in industrial settings, ranging from the manufacture of plastics and resins to solvents and chemical intermediates. Their high reactivity and ability to form a wide range of products make them invaluable in synthetic chemistry, facilitating the production of various materials and chemicals.
Medicinal and Synthetic Applications
Both aromatic and aliphatic aldehydes play crucial roles in medicinal chemistry and drug synthesis. Their structural diversity and reactivity allow for the creation of complex molecules used in drugs and medical treatments. Moreover, their applications extend to agricultural chemicals, dyes, and coatings, demonstrating their versatility and importance across multiple industries.
Frequently Asked Questions
What are aldehydes?
Aldehydes are organic compounds characterized by the presence of a carbonyl group (C=O) bonded to at least one hydrogen atom. They are key intermediates in organic synthesis and are found in various natural and synthetic products, offering a wide range of applications from the flavoring and pharmaceutical industries to material science.
How do aromatic and aliphatic aldehydes differ in structure?
Aromatic aldehydes feature a carbonyl group attached to an aromatic ring, contributing to their stability and distinctive aromas. In contrast, aliphatic aldehydes lack an aromatic ring and generally have simpler, straight or branched carbon chain structures, affecting their reactivity and physical properties.
What makes aromatic aldehydes unique?
Aromatic aldehydes are unique because of their stability, enhanced by the aromatic ring, and their notable fragrances. These properties make them preferred components in the manufacturing of perfumes, flavorings, and certain pharmaceuticals, capitalizing on their pleasant scents and chemical resilience.
Are aliphatic aldehydes more reactive than aromatic ones?
Yes, aliphatic aldehydes are typically more reactive than aromatic aldehydes. This increased reactivity stems from the lack of an aromatic ring, making them more prone to undergo nucleophilic attacks and participate in a broader range of chemical reactions, which is advantageous in various synthetic processes.
Conclusion
In conclusion, the exploration of aromatic and aliphatic aldehydes uncovers a fascinating aspect of organic chemistry that has profound implications in both nature and industry. Their contrasting properties and applications highlight the versatility of aldehydes as a whole, demonstrating how structural differences can influence chemical behavior and utility.
Understanding these differences not only enriches our knowledge of chemistry but also opens up new avenues for innovation in the creation of flavors, fragrances, and materials. As we continue to delve into the complexities of organic compounds, the study of aldehydes stands as a testament to the beauty and utility found in the molecular diversity of the world around us.