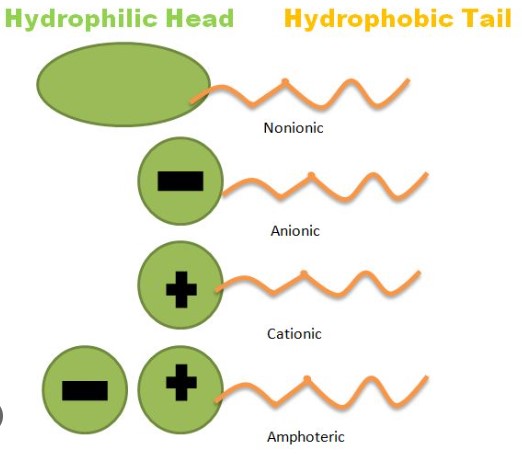
Surfactants, or surface-active agents, play a pivotal role in our daily lives and various industries, from household cleaning products to pharmaceutical formulations. These versatile compounds decrease surface tension between two liquids or a liquid and a solid, making them essential ingredients in a myriad of products we use every day.
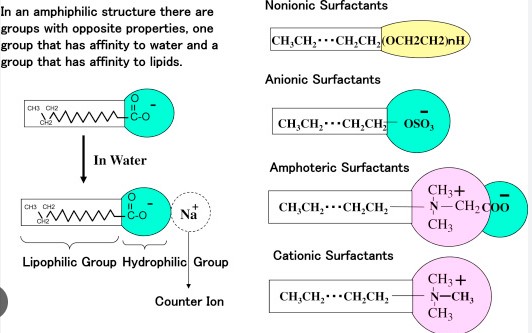
The difference between anionic, cationic, and nonionic surfactants lies primarily in their charge and molecular structure. Anionic surfactants carry a negative charge, making them effective in removing dirt and organic materials. Cationic surfactants, on the other hand, are positively charged and excel in fabric softening and antimicrobial properties. Nonionic surfactants, lacking any charge, are particularly good at breaking down substances without the ionic reaction complications, making them versatile in various formulations.
Surfactants are not just chemical compounds; they are the unsung heroes that make our soaps foamy, our clothes clean, and our shampoos effective. Their ability to interact with both water and oil makes them indispensable in creating effective, safe, and user-friendly products. Understanding their differences is not just chemical semantics but a key to unlocking their potential in everyday applications and industrial innovations.
Surfactant Basics
What Are Surfactants?
Surfactants, short for surface-active agents, are chemicals that play a critical role in cleaning, emulsifying, and stabilizing products. They have the unique ability to reduce surface tension between two substances, such as oil and water, making them indispensable in a wide range of industries.
How Surfactants Work
Surfactants operate on a simple yet profound principle. They consist of two parts: a hydrophilic (water-attracting) head and a hydrophobic (water-repelling) tail. This dual nature allows surfactants to:
- Bind to grease and dirt
- Surround the dirt, making it easier to rinse away with water
Role in Products
Surfactants are the backbone of many products we use daily, including:
- Detergents and soaps: For cleaning surfaces and laundry
- Shampoos and conditioners: To cleanse and condition hair
- Cosmetics: As emulsifiers to mix water with oils and fats
Types of Surfactants
Anionic Surfactants
Definition
Anionic surfactants carry a negative charge. They are known for their excellent cleaning abilities and are widely used in personal care and household cleaning products.
Common Uses
- Laundry detergents: To remove stains and dirt
- Dishwashing liquids: For effective grease removal
- Shampoos: For deep cleaning
Examples
- Sodium lauryl sulfate (SLS)
- Sodium dodecylbenzenesulfonate
Cationic Surfactants
Definition
Cationic surfactants have a positive charge. They excel in fabric softening and have antibacterial properties, making them valuable in personal care products and fabric care.
Common Uses
- Fabric softeners: To soften clothes
- Hair conditioners: For smooth, tangle-free hair
- Disinfectants: For their antibacterial effects
Examples
- Benzalkonium chloride
- Cetrimonium chloride
Nonionic Surfactants
Definition
Nonionic surfactants do not carry any charge. They are especially effective in formulations where ionic reactions are undesirable, making them versatile for a wide range of applications.
Common Uses
- Cosmetics: As gentle cleansers and emulsifiers
- Industrial cleaners: For non-corrosive cleaning
- Pharmaceuticals: As emulsifiers and solubilizers
Examples
- Polysorbates
- Nonoxynols
Key Differences
Charge and Structure
- Anionic: Have a negative charge; excel in removing organic stains and dirt.
- Cationic: Possess a positive charge; known for conditioning properties and antibacterial effects.
- Nonionic: No charge; prized for their mildness and versatility in formulations.
Solubility and Compatibility
Surfactants differ in their solubility (how well they dissolve in water or oil) and their compatibility with other ingredients, which affects their use in product formulations. For instance, anionic and cationic surfactants are typically not compatible with each other but can be combined with nonionic surfactants to improve a product’s performance.
Environmental Impact
The impact of surfactants on the environment depends on their:
- Biodegradability: How quickly they break down in nature. Nonionic surfactants are generally considered more biodegradable.
- Toxicity: The potential harm they may cause to aquatic life. Regulations encourage the use of surfactants with lower toxicity levels.
Applications
Household Products
Cleaning Agents
Surfactants are the heart of cleaning agents, offering properties that make soaps and detergents effective. They break down grease and lift soil from surfaces, making them easier to wash away. From dishwashing liquids to multi-purpose cleaners, surfactants ensure our homes stay clean.
Personal Care Products
In personal care, surfactants are key to creating products that not only clean but also provide a pleasant user experience. They are used in shampoos to remove oil and dirt from hair, in soaps to gently cleanse the skin, and in toothpaste to help break up plaque.
Industrial Uses
Agriculture
Surfactants play a vital role in agriculture by enhancing the effectiveness of pesticides and herbicides. They improve the spreading and absorption of products on plant surfaces, leading to better pest control and crop yield.
Pharmaceuticals
In the pharmaceutical industry, surfactants are used to improve the delivery of drugs. They can help medicines dissolve better, making them more effective. Surfactants are also used in creams and ointments to ensure the active ingredients are evenly distributed.
Textiles
Surfactants are used in the textile industry for dyeing and finishing processes. They help dyes penetrate fabrics more evenly and can improve the feel and performance of the finished product.
Selection Criteria
Product Formulation
When selecting surfactants for product formulation, two critical considerations are desired properties and cost-effectiveness. Formulators must balance the need for surfactants to perform specific functions—like foaming or emulsifying—with the overall cost of the final product.
Environmental Considerations
The environmental impact of surfactants is a growing concern. Considerations include:
- Regulations: Compliance with local and international regulations is a must.
- Sustainability: Choosing surfactants that are biodegradable and have a minimal impact on aquatic life is becoming increasingly important.
Advantages and Disadvantages
Anionic Surfactants
Pros
- Highly effective cleaners
- Cost-efficient production
- Widely available
Cons
- Potential skin irritants
- Less biodegradable than nonionic surfactants
- Can be harsh on delicate fabrics
Cationic Surfactants
Pros
- Excellent conditioning properties
- Antibacterial benefits
- Effective fabric softeners
Cons
- More expensive than anionic surfactants
- Less effective cleaners
- Toxicity to aquatic life
Nonionic Surfactants
Pros
- Mild and less irritating to the skin
- Highly biodegradable
- Stable over a wide range of pH levels
Cons
- Less effective in hard water
- More expensive than anionic surfactants
- May require higher concentrations for the same cleaning efficiency
Future Trends
Innovation in Surfactant Technology
The surfactant industry is constantly evolving, with innovation focusing on enhancing performance and reducing environmental impact. Future developments may include surfactants derived from renewable resources and technologies that improve biodegradability and reduce toxicity.
Environmental Sustainability
Sustainability is becoming a driving force in the surfactant market. Companies are investing in greener alternatives, such as surfactants made from plant-based materials, to meet consumer demand for environmentally friendly products.
Regulatory Changes
As awareness of environmental issues grows, so does the regulatory landscape. Future regulations are likely to tighten restrictions on the use of certain surfactants, pushing the industry towards more sustainable practices.
Frequently Asked Questions
What makes a surfactant anionic or cationic?
Anionic surfactants carry a negative charge in their hydrophilic (water-attracting) part, making them highly effective at removing dirt and stains. Cationic surfactants have a positive charge, which helps them adhere to negatively charged surfaces, making them excellent for conditioning fabrics and hair.
Are nonionic surfactants better for the environment?
Nonionic surfactants are often considered more environmentally friendly because they generally have lower toxicity levels and are more biodegradable than their ionic counterparts. However, the environmental impact of any surfactant depends on its specific chemical structure, usage, and disposal methods.
How do I choose the right surfactant for my product?
Selecting the right surfactant depends on the product’s intended use, desired properties, and environmental considerations. Factors such as solubility, foaming capacity, and compatibility with other ingredients play critical roles. Additionally, regulatory requirements and sustainability goals can influence the choice of surfactants in product formulations.
Can surfactants be mixed to enhance product performance?
Yes, surfactants can be mixed to enhance product performance. Combining different types of surfactants can optimize cleaning efficiency, foam production, and conditioning properties. However, careful formulation is necessary to ensure the surfactants are compatible and effective when combined.
Conclusion
Understanding the differences between anionic, cationic, and nonionic surfactants is crucial for anyone involved in creating or using detergents, cleaners, and personal care products. Each type of surfactant has its unique properties and applications, making them suitable for specific tasks. By leveraging the characteristics of these surfactants, formulators can develop products that are not only effective but also environmentally responsible.
The future of surfactant development lies in innovation, sustainability, and environmental stewardship. As we move forward, the emphasis will be on creating surfactants that offer superior performance while minimizing environmental impact. This balance is not just beneficial for consumers and industries but is essential for the well-being of our planet.