The nitrogen cycle, a cornerstone of our planet’s ecosystem, involves several biochemical processes that convert nitrogen into various forms usable by living organisms. Among these, anammox and denitrification stand out as critical yet distinctly different pathways for nitrogen conversion. These processes play pivotal roles in controlling the nitrogen balance in natural and man-made ecosystems, influencing everything from soil fertility to water quality.
Anammox and denitrification are biological processes that help remove nitrogen from the environment. Anammox, a contraction of anaerobic ammonium oxidation, is a process where specific bacteria convert ammonium and nitrite into nitrogen gas under anaerobic conditions. Denitrification, on the other hand, is the reduction of nitrates back into nitrogen gas, primarily under anaerobic conditions, facilitated by a different set of bacteria. Both serve the purpose of nitrogen removal but through distinct pathways and organisms.
The significance of these processes extends beyond the microbial world, affecting global nitrogen cycles and ecosystems. They influence agricultural practices, wastewater treatment technologies, and even the global climate by affecting greenhouse gas emissions. Understanding the differences between anammox and denitrification, including their mechanisms, environmental impacts, and applications, is essential for advancing ecological research, improving environmental management strategies, and tackling challenges such as water pollution and climate change.
Basic Concepts
Nitrogen Cycle: Role in Ecosystem
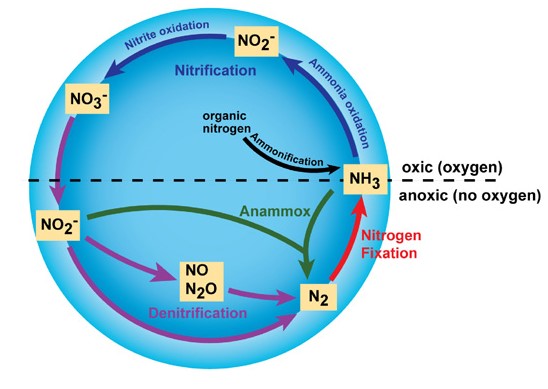
The nitrogen cycle is a fundamental part of Earth’s ecosystem. It’s the process by which nitrogen is converted into various chemical forms as it circulates among the atmosphere, terrestrial, and marine ecosystems. This cycle includes several key processes: nitrogen fixation, nitrification, denitrification, and anammox, each playing a critical role in making nitrogen available in forms that can be utilized by living organisms to synthesize vital organic compounds like DNA and proteins.
Anammox: Definition and Discovery
Anammox, short for anaerobic ammonium oxidation, is a process where nitrogen compounds are converted directly into nitrogen gas (N2) by bacteria in the absence of oxygen. Discovered in the 1990s, this process was a significant addition to the nitrogen cycle, revealing a previously unknown pathway for nitrogen gas production in natural environments. The discovery of anammox bacteria in wastewater treatment plants underscored their potential in reducing nitrogen pollution efficiently and cost-effectively.
Denitrification: Definition and Process
Denitrification is the process by which nitrates (NO3−) are reduced to nitrogen gas (N2), usually under anaerobic conditions. This process is crucial for removing excess nitrates from soil and water, preventing eutrophication, and maintaining the balance of nitrogen in the ecosystem. Denitrification is facilitated by a variety of microorganisms and is an essential component of the nitrogen cycle, complementing the nitrogen fixation process.
Key Differences
Biological Process
Anammox Mechanism
The anammox process involves bacteria that convert ammonium (NH4+) and nitrite (NO2−) into nitrogen gas (N2). This reaction occurs inside special cellular compartments called anammoxosomes and is unique because it happens without oxygen. The anammox reaction is crucial for managing nitrogen levels in anaerobic environments, such as deep-sea sediments and freshwater and marine water columns.
Denitrification Mechanism
Denitrification, in contrast, typically involves the sequential reduction of nitrate to nitrite, nitric oxide, nitrous oxide, and finally to nitrogen gas. This process is carried out by various denitrifying bacteria under low-oxygen conditions. Denitrification helps regulate the nitrogen levels in the soil and aquatic ecosystems, preventing excess nitrate accumulation.
Environmental Impact: Contributions to Nitrogen Cycle
Both anammox and denitrification are vital for the environmental management of nitrogen. Anammox provides a direct and efficient pathway for converting ammonium and nitrite into nitrogen gas, especially in oxygen-minimal environments. Denitrification complements this by reducing nitrates in environments where oxygen is more scarce, thus maintaining a balance within the nitrogen cycle and preventing the over-accumulation of nitrogenous compounds that could lead to eutrophication.
Organisms Involved
Anammox Bacteria
Anammox bacteria belong to the Planctomycetes phylum and are known for their unique cellular structures and metabolic capabilities. They thrive in anaerobic conditions and are found in a variety of habitats, from marine and freshwater systems to soil and wastewater treatment plants.
Denitrifying Bacteria
Denitrifying bacteria are more diverse, including species from genera like Pseudomonas, Paracoccus, and Bacillus. These organisms can switch to denitrification in the absence of oxygen, making them versatile in managing nitrogen in different environmental contexts.
Anammox Process
Discovery and History: Initial Findings
The anammox process was a revolutionary discovery in the 1990s. Initially observed in wastewater treatment systems, this process opened new avenues for nitrogen management in industrial and natural settings. It challenged the traditional view of the nitrogen cycle by introducing a significant anaerobic pathway that was previously unknown.
Chemical Reactions: Anammox Reaction Formula
The chemical formula for the anammox reaction is: ��4++��2−→�2+2�2�NH4++NO2−→N2+2H2O This equation highlights the process’s efficiency in converting ammonium and nitrite directly into nitrogen gas and water, showcasing its environmental significance.
Environmental Conditions: Preferred Habitats and Conditions
Anammox bacteria prefer anaerobic (oxygen-free) environments with ample ammonium and nitrite. Such conditions are commonly found in marine sediments, anoxic water columns, and wastewater treatment facilities. Their ability to operate in these settings makes them invaluable for natural and artificial nitrogen cycling processes.
Denitrification Process
Chemical Reactions: Denitrification Reaction Formula
The denitrification process can be summarized by the following chemical reaction: ��3−→��2−→��→�2�→�2NO3−→NO2−→NO→N2O→N2 This series of reductions shows how nitrates are ultimately converted into nitrogen gas, highlighting the process’s role in mitigating nitrogen accumulation.
Environmental Conditions: Preferred Habitats and Conditions
Denitrifying bacteria are versatile and can be found in a variety of anaerobic or low-oxygen environments. These include water-logged soils, the bottom layers of lakes and oceans, and the interiors of thick biofilms. Their widespread presence underscores the global significance of denitrification in controlling nitrogen levels.
Ecological Significance
Role in Nitrogen Cycle
Anammox and denitrification are essential biological processes that help maintain the balance of the nitrogen cycle, one of Earth’s critical biogeochemical cycles. While anammox directly converts ammonium and nitrite into nitrogen gas, denitrification reduces nitrates and nitrites into nitrogen gas or, occasionally, nitrous oxide. Together, these processes prevent the accumulation of nitrogen in the environment, which can lead to harmful effects such as eutrophication.
Comparison of Contributions
Although both anammox and denitrification contribute to the nitrogen cycle, their roles differ significantly. Anammox is primarily active in anaerobic environments where oxygen is minimal, making it a key process in deep sea sediments and some freshwater systems. Denitrification, on the other hand, acts as a broader ecological safety net, occurring in a variety of settings, including soils, wetlands, and water bodies, wherever there’s a deficit of oxygen.
Environmental Impact
Effects on Water Quality and Air Pollution
Both processes have profound impacts on water quality and air pollution. By removing excess nitrogen from water bodies, they prevent eutrophication, the overgrowth of algae that depletes oxygen in water and kills aquatic life. Regarding air pollution, denitrification can produce nitrous oxide, a potent greenhouse gas, emphasizing the need for careful management of this process in agricultural fields and wastewater treatment plants.
Applications and Implications
Wastewater Treatment
In wastewater treatment, anammox and denitrification are harnessed to remove nitrogen from sewage and industrial effluents. This treatment is crucial for preventing the discharge of nutrient-rich waters into natural bodies, which could otherwise trigger eutrophication. Anammox, with its lower energy demand and absence of a need for organic carbon, offers a cost-effective alternative to traditional nitrogen removal processes.
Agriculture
In agriculture, the understanding and management of these processes can significantly impact fertilization and soil health. Denitrification can lead to the loss of valuable nitrate fertilizers as nitrogen gas, prompting the need for more efficient use of fertilizers. Meanwhile, the anammox process has yet to be directly applied in agricultural settings but has implications for managing waterlogged soils and reducing runoff.
Climate Change
The contribution to greenhouse gas emissions is a critical aspect of both processes. While anammox is generally not associated with significant greenhouse gas production, denitrification can emit nitrous oxide. This aspect makes the study and management of denitrification especially important in the context of climate change, as reducing nitrous oxide emissions can have a significant impact on global warming potential.
Challenges and Opportunities
Technological Advances
Recent technological advances have opened new possibilities for harnessing anammox and denitrification. Innovations in bioreactor designs and the development of more efficient microbial strains promise to enhance the applicability and efficiency of these processes in both wastewater treatment and potentially agriculture. The integration of these technologies into existing systems poses a significant opportunity for environmental sustainability.
Environmental Challenges
However, there are environmental challenges associated with the implementation of these processes in natural settings. One of the main issues is the variability of environmental conditions, which can affect the efficiency and reliability of anammox and denitrification. Additionally, the potential production of nitrous oxide during denitrification raises concerns about its contribution to greenhouse gas emissions and climate change.
Frequently Asked Questions
What is Anammox?
Anammox, short for anaerobic ammonium oxidation, is a biological process in which bacteria convert ammonium (NH4+) and nitrite (NO2−) directly into nitrogen gas (N2) in the absence of oxygen. This process provides an energy-efficient and cost-effective method for removing nitrogen from wastewater and plays a crucial role in the global nitrogen cycle by naturally limiting the accumulation of nitrogen in aquatic ecosystems.
How does Denitrification work?
Denitrification is a stepwise process where bacteria convert nitrate (NO3−) into nitrogen gas (N2) or nitrous oxide (N2O) through intermediate products such as nitrite (NO2−), nitric oxide (NO), and nitrous oxide (N2O). This process predominantly occurs under anaerobic conditions, where oxygen is scarce, making it vital for managing nitrogen levels in water bodies and soils, thus preventing excessive nutrient buildup and eutrophication.
Why are Anammox and Denitrification important?
Anammox and denitrification are integral to the nitrogen cycle, playing essential roles in removing excess nitrogen from environments, such as water bodies and soil. Their importance lies in preventing the accumulation of nitrogen that can lead to environmental issues like eutrophication, which causes harmful algal blooms and dead zones in aquatic ecosystems. Additionally, they are key processes in wastewater treatment, helping to reduce nitrogen pollution and improve water quality.
Can Anammox and Denitrification affect climate change?
Yes, anammox and denitrification can influence climate change. Both processes contribute to the natural cycling of nitrogen and can emit nitrous oxide (N2O), a potent greenhouse gas, as a byproduct. Understanding and managing these biological processes can help mitigate N2O emissions from agricultural practices and wastewater treatment, thereby contributing to climate change mitigation efforts.
Conclusion
Anammox and denitrification represent vital cogs in the wheel of the nitrogen cycle, each playing a unique role in maintaining the balance of nitrogen in our environment. By converting excess nitrogen compounds into nitrogen gas, these processes help mitigate the risk of eutrophication in aquatic ecosystems, improve the quality of our water sources, and even play a role in climate regulation through the management of greenhouse gas emissions.
Their significance extends beyond their environmental impact, influencing agricultural practices, wastewater treatment, and our understanding of microbial ecology. As research continues to uncover the intricacies of these processes, it becomes increasingly clear how crucial they are for sustaining life on Earth, highlighting the need for continued study and responsible environmental management practices.