When it comes to the study of acids and bases, two essential terms that come into play are amphiprotic and amphoteric. In this blog post, we will be looking at the differences between these two terms and why it is helpful to know the difference.
We will explore what makes them unique and what the implications are for their use in chemistry. By the end of this article, you should have a better understanding of the differences between amphiprotic and amphoteric and how they can be used in chemistry.
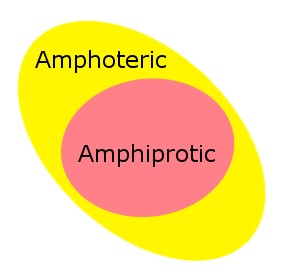
Overview of amphiprotic and amphoteric
Amphiprotic and amphoteric molecules have a lot in common, but there are some key differences that set them apart. Amphiprotic molecules are capable of donating and accepting protons, while amphoteric molecules can both donate and accept protons, but they’re also able to neutralize themselves in the presence of a base or an acid.
Because of this, amphoteric molecules have the ability to act as both an acid and a base, while amphiprotic molecules can only donate or accept protons. This makes amphoteric molecules much more versatile as they can react with different types of substances.
Difference between amphiprotic and amphoteric
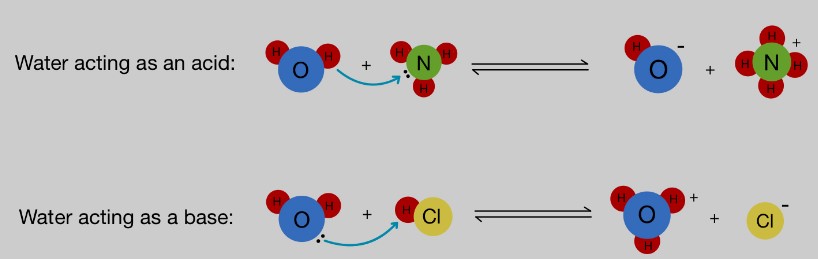
When discussing chemistry, two terms that often come up are “amphiprotic” and “amphoteric. ” These terms refer to the way molecules interact with acids and bases. In short, amphiprotic molecules can act as acids or bases, while amphoteric molecules can act as either an acid or a base depending on the other substances present.
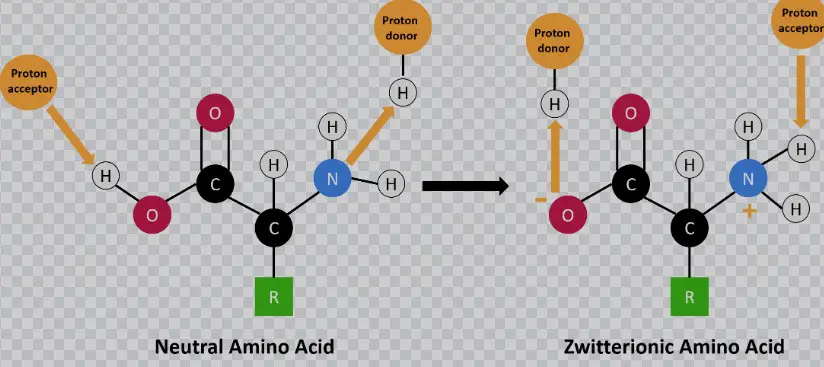
Amphiprotic molecules contain both acidic and basic functional groups and can therefore either donate or accept a proton. Examples of amphiprotic molecules include proteins, amino acids, and water.
On the other hand, amphoteric molecules contain both acidic and basic functional groups, but they can only donate or accept a proton depending on the other substances present. Examples of amphoteric molecules include alcohols, phenols, and amines. In summary, the major difference between amphiprotic and amphoteric molecules is that amphiprotic molecules can act as both an acid and a base, while amphoteric molecules can only act as one or the other depending on the other substances present.
In summary, the major difference between amphiprotic and amphoteric molecules is that amphiprotic molecules can act as both an acid and a base, while amphoteric molecules can only act as one or the other depending on the other substances present.
Examples of amphiprotic and amphoteric compounds
Amphiprotic and amphoteric compounds are two classifications of chemical compounds that differ in their ability to either donate or accept protons. Amphiprotic compounds are able to both donate and accept protons, while amphoteric compounds can only donate or accept protons depending on the conditions. A common example of an amphiprotic compound is water, which can donate protons in the form of hydronium ions and accept protons to form hydroxide ions.
An example of an amphoteric compound is ammonia, which can donate protons to form ammonium ions under acidic conditions and accept protons to form hydroxide ions under basic conditions. In general, amphiprotic compounds are more versatile than amphoteric compounds, as they can act as both an acid and a base.
Applications of amphiprotic and amphoteric compounds
Amphiprotic and amphoteric compounds are both compounds containing both acidic and basic properties. The difference between the two lies in their ability to react with both acids and bases. Amphiprotic compounds, such as water, are able to react with both acids and bases, donating or accepting protons as needed.
Amphoteric compounds, on the other hand, can react with both acids and bases, but not in the same way. Amphoteric compounds can donate or accept protons depending on the environment, and the pH of the solution.
Applications of amphiprotic and amphoteric compounds are vast, and include many everyday items. In medicine, amphiprotic compounds are used as buffers, to maintain a stable pH in the body, and amphoteric compounds are used as antacids to reduce acidity in the stomach. In the food industry, amphiprotic compounds are used to adjust the pH in various products, and amphoteric compounds are used as emulsifying agents.
In cosmetics, amphiprotic compounds are used to adjust the pH of the skin, and amphoteric compounds are used as cleansing agents. Overall, amphiprotic and amphoteric compounds are essential compounds used in many different applications.
Understanding the difference between the two is key, as each compound has its own unique properties and applications.
Pros and cons of amphiprotic and amphoteric compounds
Amphiprotic and amphoteric compounds both contain hydrogen atoms, but the way they behave in aqueous solutions can be quite different. Amphiprotic compounds can act as either an acid or a base, depending on the pH of the solution they are in, whereas amphoteric compounds can react with both acids and bases. This means that amphiprotic compounds have the ability to either donate or accept protons, while amphoteric compounds can do both.
This means that amphiprotic compounds have the ability to either donate or accept protons, while amphoteric compounds can do both. The major difference between the two is that amphiprotic compounds can only act as either an acid or a base, while amphoteric compounds can act as either an acid or a base, depending on the pH of the solution. This makes amphoteric compounds more versatile in terms of their reactivity.
On the other hand, amphiprotic compounds are more stable than amphoteric compounds, meaning that they are less likely to react with other compounds in aqueous solutions.
Final Touch
In conclusion, the difference between amphiprotic and amphoteric molecules is that amphiprotic molecules can both donate and accept protons while amphoteric molecules can either donate or accept protons depending on the environment. Amphiprotic molecules are more common in nature than amphoteric molecules. Both types of molecules can act as both acids and bases, and can be used to balance out solutions.
Both types of molecules can act as both acids and bases, and can be used to balance out solutions.