Aluminates and meta-aluminates play a pivotal role in a multitude of industrial applications, yet their distinct characteristics and implications are not commonly understood. Both compounds are derivatives of aluminum and oxygen but differ significantly in their chemical structure and physical properties. This differentiation not only influences their utility in various sectors but also their production and environmental impact.
Aluminate is typically formed when aluminum oxide reacts with alkalis, presenting a basic compound, while meta-aluminate is produced under different conditions, offering a varied chemical structure. This basic distinction is crucial for professionals in chemistry and industry to grasp in order to effectively apply these materials in practical settings.
Exploring these compounds reveals a fascinating aspect of industrial chemistry that impacts everyday products from water treatment systems to building materials. The nuanced differences between aluminate and meta-aluminate affect everything from their solubility and toxicity to their environmental footprint, making a thorough understanding essential for any application involving these substances.
Basic Chemistry
What is Aluminate?
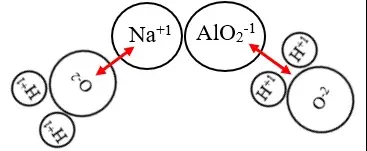
Aluminate compounds form when aluminum oxide (Al₂O₃) reacts with basic substances such as hydroxides. These are ionic compounds, primarily consisting of aluminum ions and oxide ions, bound together in a lattice structure. Aluminates are known for their ability to form complex solutions with water, making them highly valuable in various industrial processes.
What is Meta-Aluminate?
Meta-aluminate differs from traditional aluminate by its formation and chemical behavior. It is typically produced under conditions where aluminum oxide interacts with stronger bases, resulting in a distinct stoichiometric ratio. Meta-aluminates are less soluble in water compared to their aluminate counterparts but offer greater stability at high temperatures.
Structural Differences
Chemical Composition
Aluminates generally have the formula Al₂O₃.xH₂O, where x denotes water molecules that vary depending on the specific aluminate compound. In contrast, meta-aluminates often form more complex structures and can include additional elements like sodium or potassium, leading to formulas such as NaAlO₂ or KAlO₂.
Molecular Structure
The molecular structure of aluminates features a network of aluminum and oxygen atoms bonded in a robust lattice. This structure is somewhat flexible, allowing for various hydrates to form. Meta-aluminates, however, typically exhibit a more rigid and less hydrated lattice, which contributes to their unique properties and uses.
Production Processes
Synthesis of Aluminate
- Mixing materials: Start by combining aluminum hydroxide with a strong base like sodium hydroxide.
- Heating: Heat the mixture to facilitate the reaction, forming sodium aluminate.
- Cooling and settling: Allow the mixture to cool. Aluminate crystals will form and settle.
- Filtering: Filter out the crystals and wash them to remove impurities.
Synthesis of Meta-Aluminate
- Base preparation: Prepare a more concentrated base solution than used for aluminate.
- Mixing at high temperature: Introduce aluminum oxide to the base at a higher temperature to promote meta-aluminate formation.
- Maintaining temperature: Keep the temperature steady to ensure the formation of meta-aluminate rather than aluminate.
- Cooling and collection: Cool the solution and collect the meta-aluminate that precipitates.
Physical Properties
Solubility
Aluminates are relatively soluble in water, which is crucial for their application in water treatment and similar processes. This solubility varies significantly with the pH of the solution and the specific type of aluminate. Meta-aluminates, by comparison, are much less soluble, particularly in neutral or acidic environments, limiting their use to more niche applications.
Density and Hardness
The density and hardness of aluminate compounds depend largely on their composition and molecular structure. Aluminates, being more hydrated, are generally less dense and softer than meta-aluminates. The latter’s denser and more crystalline structure lends it greater hardness, making it suitable for use in refractory materials and other applications requiring high thermal stability.
Applications in Industry
Role in Water Treatment
Aluminates are crucial in water treatment processes due to their ability to help in coagulation and flocculation. These processes are vital for removing impurities and clarifying water, which is essential for both municipal and industrial uses. The mechanism involves aluminate reacting with water impurities, forming flocs that are large enough to be removed by sedimentation or filtration.
- Coagulation step: Aluminate salts are added to water to neutralize the charge of dissolved particles.
- Flocculation step: These neutralized particles bind together, forming larger aggregates.
- Removal: The aggregates settle down or are filtered out, leading to cleaner water.
Uses in Cement and Ceramics
In the production of cement, aluminates contribute to the material’s hydraulic properties, which are necessary for the cement to set and harden under water. Aluminates react with water to form compounds that impart strength and durability to cement, essential for construction.
- Cement hydration: Aluminates react with water during the curing process, improving the strength and stability of the cement.
- Ceramics production: In ceramics, meta-aluminates are used due to their thermal stability and ability to withstand high temperatures without decomposing.
Environmental Impact
Safety Measures
Handling aluminates requires specific safety measures to protect both the workers and the environment. Exposure to aluminate dust can cause respiratory issues, skin irritation, and other health problems.
- Respiratory protection: Workers should wear masks to avoid inhaling aluminate powder.
- Skin protection: Gloves and protective clothing are necessary to prevent skin contact.
- Proper ventilation: Facilities must be well-ventilated to disperse any airborne particles quickly.
Recycling and Disposal
The disposal of aluminate involves measures to prevent environmental contamination. Recycling of aluminate materials is also becoming more prevalent, reducing the need for raw materials and minimizing waste.
- Recycling: Used aluminate from industrial processes can be converted into other products or reused in different applications.
- Safe disposal: Non-recyclable aluminate waste must be treated and disposed of in accordance with environmental regulations to prevent any potential harm.
Market Trends
Demand and Supply Dynamics
The demand for aluminates is influenced by global trends in water treatment, construction, and environmental regulations. Supply dynamics are impacted by the availability of raw materials, such as bauxite, and the operational capacities of aluminate producers.
- Increasing demand: Growing global urbanization and infrastructure projects drive demand for cement and, consequently, aluminates.
- Supply constraints: Fluctuations in the supply of raw materials can affect aluminate production costs and availability.
Future Outlook
The future of aluminate and meta-aluminate usage looks promising due to ongoing research and development in the materials science field. Innovations in recycling technologies and the development of more environmentally friendly aluminate compounds are likely to enhance their market appeal.
- Innovation in applications: New uses for aluminates in technology and environmental applications are being explored.
- Environmental sustainability: The focus on reducing environmental impact is driving improvements in aluminate production and utilization processes.
FAQs
What is Aluminate?
Aluminate refers to a compound composed of an aluminum ion and oxygen ions, typically found in environments where aluminum reacts with basic substances. It is used extensively in water purification and as an additive in the building industry.
How is Meta-Aluminate different?
Meta-aluminate, often more complex in structure, forms under conditions that favor a different stoichiometry from typical aluminates. This difference in chemical makeup allows for unique applications in high-temperature processes and specialized ceramics.
Where are these compounds used?
Both aluminates and meta-aluminates are integral to several industrial processes. Aluminates are crucial in cement production and water treatment, whereas meta-aluminates find their use in specialized ceramic materials and certain chemical syntheses.
What are the environmental impacts of Aluminates?
The production and use of aluminates can have environmental implications, including the disposal of industrial waste and the management of chemical residues. However, advancements in recycling technologies have begun to mitigate these impacts.
Conclusion
In conclusion, the nuanced differences between aluminate and meta-aluminate are not merely academic but have practical implications that influence their applications across various industries. Their unique properties dictate their utility in fields as diverse as construction and environmental management.
Understanding these compounds enables industry professionals to optimize their use and mitigate associated environmental impacts effectively. As the demand for more sustainable industrial practices grows, the role of aluminates and meta-aluminates in achieving these goals becomes increasingly significant.

