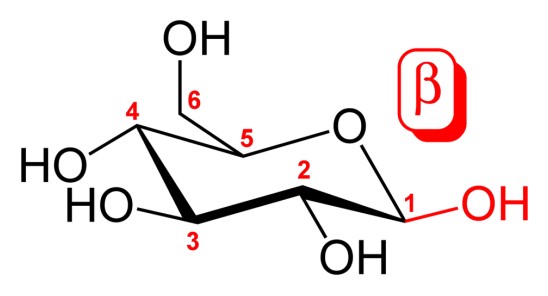
Carbohydrates, the most abundant biomolecules on earth, play crucial roles in biological systems and are essential components of our diet. Central to understanding their biological importance is the concept of anomers, specific forms of carbohydrates that differ only in their spatial configuration. This subtle difference can significantly impact their chemical behavior and biological functions.
Anomers are types of stereoisomers, specifically differing at the carbonyl carbon atom after the formation of a cyclic structure. The alpha and beta anomers are distinct in that the alpha anomer has the -OH group on the anomeric carbon trans (opposite) to the -CH2OH side group, whereas the beta anomer has it cis (same side) to the -CH2OH group. This configuration affects not only their structure but also their reactivity and interaction with other molecules.
The study of these molecules extends beyond simple academic curiosity; it has practical applications in fields ranging from biochemistry to food science. Anomers’ behavior influences the taste, texture, and stability of food products, and plays a critical role in the efficacy and safety of pharmaceutical agents.
Carbohydrate Basics
Structure of Carbohydrates
Carbohydrates are organic compounds composed of carbon, hydrogen, and oxygen, typically in a ratio of 1:2:1. This fundamental makeup allows carbohydrates to serve as major energy sources and structural components in living organisms. The basic structure of carbohydrates ranges from small sugar molecules like glucose to large polysaccharides like starch.
Monosaccharides and Their Forms
Monosaccharides are the simplest form of carbohydrates, consisting of single sugar units with the general formula (CH2O)n, where n can be three or more. Glucose, the most common monosaccharide, is a prime energy source for cells. Monosaccharides can exist in different forms based on their chemical structure:
- Linear form: The open-chain form of monosaccharides with a backbone of carbon atoms.
- Ring form: When dissolved in water, monosaccharides tend to form ring structures, which are more chemically stable.
Anomers Defined
Concept of Anomeric Carbon
The anomeric carbon is a key concept in carbohydrate chemistry. It is the carbon atom that becomes a new chiral center when monosaccharides cyclize to form ring structures. The orientation of the hydroxyl group attached to this carbon defines whether a sugar is in the alpha or beta form.
Anomer Formation Process
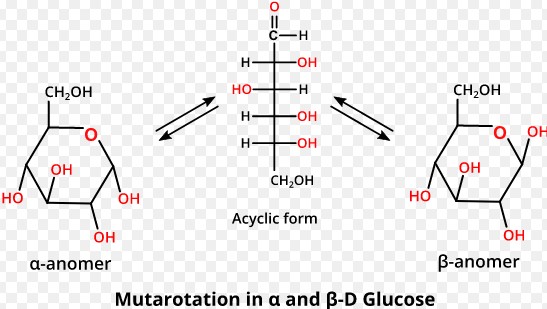
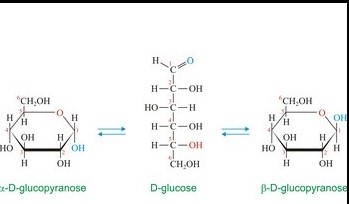
Anomers are formed through a process known as ring closure, where the linear form of a sugar molecule reacts with itself to form a cyclic structure. This process creates a new asymmetric carbon atom, the anomeric carbon, which can have either of two configurations:
- Alpha (α): The hydroxyl group is opposite to the CH2OH group.
- Beta (β): The hydroxyl group is on the same side as the CH2OH group.
Alpha Anomers
Structural Characteristics
Alpha anomers are characterized by the position of the hydroxyl group at the anomeric carbon, which points in the opposite direction from the main molecule chain. This positioning affects the molecule’s stability and reactivity.
Common Examples
Glucose in its alpha form (α-glucose) is a prevalent example. It is less soluble in water than its beta counterpart and has different crystalline structures and melting points.
Role in Biology and Chemistry
Alpha anomers play significant roles in biology, particularly in energy storage. Starch, a storage form of glucose in plants, primarily consists of alpha-linked glucose units. This linkage makes the starch less reactive, which is ideal for storage purposes.
Beta Anomers
Structural Characteristics
In beta anomers, the hydroxyl group at the anomeric carbon is oriented in the same direction as the CH2OH side chain. This structural orientation generally results in greater solubility and different physical properties compared to alpha anomers.
Common Examples
Beta-glucose is a common example of a beta anomer. It is more soluble in water than alpha-glucose, which impacts its biological functionality and its use in various industrial applications.
Role in Biology and Chemistry
Beta anomers are crucial in the formation of cellulose, the primary structural component of plant cell walls. The beta linkages in cellulose confer rigidity and resistance to hydrolysis, which are vital for maintaining the structural integrity of plant tissues. This characteristic also makes cellulose a challenging material to convert into biofuels, as its beta linkages are resistant to enzymes that break down alpha linkages.
Comparing Alpha and Beta Anomers
Differences in Structure
Alpha and beta anomers differ primarily at the placement of the hydroxyl group on the anomeric carbon. This seemingly minor structural variation leads to distinct three-dimensional forms. Alpha anomers have the hydroxyl group oriented axially, away from the ring’s skeleton, whereas beta anomers feature the hydroxyl group equatorially, aligning with the ring structure. This orientation affects both the stability and the reactivity of the sugar molecules.
Chemical Properties
The chemical behaviors of alpha and beta anomers can vary significantly:
- Reactivity: Alpha anomers are typically less reactive compared to beta anomers due to the axial position of their hydroxyl group, which results in greater steric hindrance.
- Tendency to Mutarotate: Beta anomers generally mutarotate more readily than alpha anomers, reflecting their higher solubility and less hindered structure.
Physical Properties
- Solubility: Beta anomers tend to be more soluble in water due to their more favorable interaction with water molecules.
- Melting Point: The different orientations of hydroxyl groups also influence the melting points of alpha and beta anomers, with beta forms usually having slightly lower melting points due to their less ordered crystalline structure.
Biological Significance
Impact on Enzyme Interaction
Enzymes that degrade or modify carbohydrates typically have a high specificity for the anomer of the substrate. For example, certain digestive enzymes in the human body specifically target alpha-linked glucose polymers like starch, but not beta-linked polymers like cellulose.
Digestibility Differences
- Alpha Anomers: Easily digested by enzymes such as amylase, which breaks down starch into glucose for absorption.
- Beta Anomers: Resistant to digestive enzymes, which makes substances like cellulose a dietary fiber that aids in digestive health but is not used directly for energy.
Relevance in Pharmaceuticals
The anomer form can influence the efficacy and safety of carbohydrate-based drugs. For instance, beta anomers might be preferred in drug design due to their stability and prolonged activity in the biological system compared to the more reactive alpha forms.
Industrial Applications
Use in Food Products
Alpha and beta anomers find diverse applications in the food industry:
- Alpha Anomers: Commonly used in the production of bread and other baked goods where their rapid enzymatic breakdown is desired.
- Beta Anomers: Often used as dietary fibers in health foods due to their resistance to digestion, contributing to a lower glycemic index.
Applications in Medicine
In medicine, the specific properties of anomers are utilized for targeted therapeutic effects:
- Beta Anomers: Utilized in the creation of sustained-release drug formulations, benefiting from their structural stability.
Analytical Techniques
Methods to Differentiate Anomers
Identifying and differentiating between alpha and beta anomers is crucial for both academic research and industrial applications. The primary methods include:
- Polarimetry: Measures the rotation of plane-polarized light by a substance, which differs between anomers.
- Melting Point Analysis: Helps in distinguishing anomers through their different melting points.
Spectroscopy and Chromatography
- NMR Spectroscopy (Nuclear Magnetic Resonance): Powerful in identifying the specific atomic structure and arrangement in anomers by observing the behavior of nuclei in a magnetic field.
- HPLC (High-Performance Liquid Chromatography): Separates anomers based on their solubility and interaction with the chromatographic medium, useful for purifying and quantifying the amounts of each anomer in a mixture.
Frequently Asked Questions
What is an anomer?
An anomer is a type of stereoisomer found in carbohydrate chemistry, differing from other forms due to the orientation of the substituent at the anomeric carbon in a saccharide molecule. This small structural change can significantly affect the sugar’s properties and its biological or industrial use.
How do alpha and beta anomers differ?
Alpha and beta anomers differ in the orientation of the hydroxyl group attached to the anomeric carbon of a sugar molecule. In the alpha anomer, the hydroxyl group is oriented opposite to the CH2OH group, whereas in the beta anomer, it is on the same side.
Why are anomers important in biology?
Anomers play a critical role in biology because their different structures interact differently with enzymes and other molecules. This interaction can affect the digestion of carbohydrates, their metabolic pathways, and their role in various biological processes.
Can anomers change forms?
Yes, under certain conditions, anomers can interconvert through a process known as mutarotation, where they shift between the alpha and beta forms. This transformation occurs in aqueous solutions and involves the temporary opening of the ring structure.
Conclusion
Understanding the difference between alpha and beta anomers is more than an academic pursuit; it has direct implications for numerous scientific fields and industries. The distinct properties of each anomer affect everything from how sugars are metabolized in our bodies to how they are used in pharmaceutical formulations.
This exploration not only underscores the complexity of seemingly simple sugar molecules but also highlights the importance of stereochemistry in the real world. The ongoing study of anomers continues to reveal intricate details that could lead to advancements in food science, medicine, and beyond.