In the realm of organic chemistry, alkoxides and phenoxides represent two fundamentally important classes of compounds, each with distinct characteristics and applications. These compounds play crucial roles in various chemical reactions, serving as intermediates in synthesis processes or as catalysts enhancing reaction rates. Their importance extends beyond academic interest, influencing the production of materials, pharmaceuticals, and other chemicals in the industrial sector.
Alkoxides are compounds formed by the replacement of a hydrogen atom in an alcohol molecule with a metal, while phenoxides arise similarly from phenols. The key difference between alkoxides and phenoxides lies in their structural backbone – alkoxides are derived from aliphatic alcohols, whereas phenoxides stem from aromatic phenols. This fundamental distinction leads to variations in their chemical behavior, reactivity, and applications, making an understanding of their differences essential for chemists and industry professionals alike.
Exploring the distinctions between alkoxides and phenoxides reveals insights into their stability, reactivity, and practical applications. The nature of the alkyl group in alkoxides influences their solubility and reactivity, whereas the aromatic structure of phenoxides introduces unique properties due to resonance stabilization. Such differences underscore the importance of these compounds in synthetic chemistry, environmental considerations, and technological advancements.
Basic Concepts
Alkoxides
Definition and General Structure
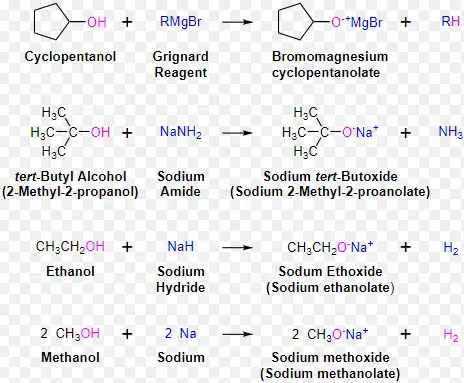
Alkoxides are organic compounds characterized by an alkoxide ion (RO-), where ‘R’ represents an alkyl group connected to an oxygen atom. These ions form when the hydrogen atom of an alcohol’s hydroxyl group (-OH) is replaced by a metal, typically sodium (Na) or potassium (K), resulting in a negative charge on the oxygen atom.
Formation and Examples
Alkoxides are primarily formed through the reaction of alcohols with metallic bases. For example:
- Sodium or potassium reacts with an alcohol, releasing hydrogen gas and forming the alkoxide.
Examples of alkoxides include sodium methoxide (NaOCH₃) and potassium tert-butoxide (KOtBu), widely used in organic synthesis due to their strong nucleophilic properties.
Phenoxides
Definition and General Structure
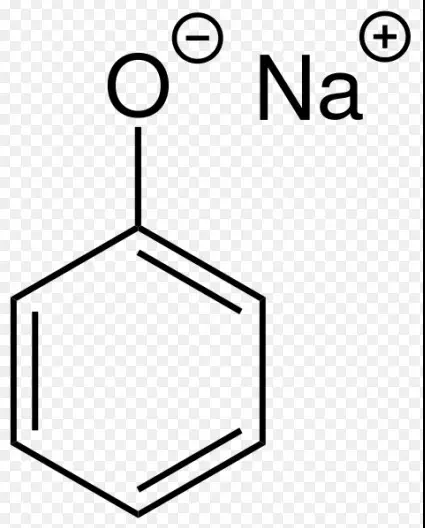
Phenoxides are similar to alkoxides but are derived from phenols. They contain a phenoxide ion (ArO-), where ‘Ar’ stands for an aryl group linked to an oxygen atom. This configuration arises when a phenol’s hydrogen is substituted by a metal, giving the oxygen a negative charge.
Formation and Examples
Phenoxides form when phenols react with metals like sodium or potassium. The process is akin to alkoxide formation but involves the deprotonation of a phenol.
Examples include sodium phenoxide (NaOC₆H₅), utilized in the production of various chemicals, demonstrating the utility of phenoxides in organic chemistry.
Chemical Properties
Stability
Comparison of Stability in Alkoxides vs. Phenoxides
Alkoxides and phenoxides exhibit different levels of stability, mainly due to the electronic effects involved. Phenoxides tend to be more stable than alkoxides because of the resonance stabilization provided by the aromatic ring in phenols. This resonance allows the negative charge on the oxygen atom to delocalize over the aromatic system, reducing the compound’s reactivity.
Factors Influencing Stability
Several factors affect the stability of these compounds, including:
- Electronegativity of the metal: Metals with higher electronegativity tend to form more stable compounds.
- Resonance effects: As mentioned, resonance in phenoxides increases their stability.
- Steric factors: The size and arrangement of the alkyl groups in alkoxides can influence their stability by hindering or facilitating reactions.
Reactivity
Alkoxide Reactivity Patterns
Alkoxides are highly reactive, primarily acting as strong bases and nucleophiles. They readily engage in:
- Nucleophilic substitution reactions
- Esterification reactions
- Williamson ether synthesis
Phenoxide Reactivity Patterns
Phenoxides, while stable due to resonance, also participate in nucleophilic substitution, especially in aromatic substitution reactions where the phenoxide ion acts as a nucleophile.
Comparative Analysis
The key difference in reactivity stems from the stabilization effects in phenoxides, which alkoxides lack. This distinction means phenoxides are less prone to react in conditions where alkoxides would readily participate.
Physical Properties
Solubility
Alkoxide Solubility in Various Solvents
Alkoxides are generally soluble in polar solvents like water, methanol, and ethanol. The solubility depends on the:
- Length of the alkyl chain: Shorter chains increase solubility.
- Polarity of the solvent: More polar solvents dissolve alkoxides more efficiently.
Phenoxide Solubility in Various Solvents
Phenoxides show good solubility in polar solvents as well, but their aromatic nature also allows for solubility in some less polar solvents.
Factors Affecting Solubility
- Polarity of the compound and solvent
- Hydrogen bonding capability
- Ionic nature of the compound
Boiling and Melting Points
Trends in Alkoxides
Alkoxides generally have lower boiling and melting points compared to phenoxides, attributable to their less extensive hydrogen bonding and lower molecular weight.
Electronic Structure
Orbital Configuration
Alkoxide Orbital Analysis
Alkoxides, with their simple molecular structure, feature an oxygen atom bonded to both an alkyl group and a metal ion. The oxygen atom in alkoxides has two lone pairs of electrons, residing in sp3 hybrid orbitals. These lone pairs play a crucial role in the reactivity of alkoxides, making them effective nucleophiles. The metal ion, typically alkali like sodium or potassium, interacts with the oxygen’s lone pairs, forming a coordinate covalent bond. This interaction significantly affects the alkoxide’s electronic properties and reactivity, particularly in synthesis reactions.
Phenoxide Orbital Analysis
Phenoxides, on the other hand, exhibit a more complex electronic structure due to the conjugation within the aromatic ring. The oxygen atom in phenoxides also possesses two lone pairs, but one of these pairs contributes to resonance with the aromatic system. This delocalization over the aromatic ring results in a partially double bond character between oxygen and the carbon it’s attached to, which stabilizes the phenoxide ion. The resonance effect profoundly influences the phenoxide’s chemical behavior, making it a less reactive nucleophile than its alkoxide counterparts.
Impact on Properties and Reactivity
The orbital configurations of alkoxides and phenoxides directly impact their chemical reactivity. Alkoxides, lacking significant resonance stabilization, are more reactive, especially in conditions favoring nucleophilic attacks. Phenoxides, with their resonance-stabilized structure, show enhanced stability and selectivity in reactions, particularly those involving electrophiles.
Resonance Effects
Role in Phenoxides
Resonance effects in phenoxides increase their stability by delocalizing the negative charge over the aromatic ring. This delocalization reduces the electron density on the oxygen atom, making phenoxides less reactive towards electrophilic reagents. Moreover, the resonance stabilization influences the acidic strength of phenols, from which phenoxides are derived, making them stronger acids compared to aliphatic alcohols.
Comparison with Alkoxides
Alkoxides lack the extensive resonance stabilization seen in phenoxides. As a result, alkoxides are more reactive and serve as stronger bases and nucleophiles. This difference in resonance effects between alkoxides and phenoxides is a fundamental aspect that influences their use in organic synthesis and reactivity patterns.
Applications
Industrial Uses
Alkoxides in Synthesis and Manufacturing
Alkoxides play a pivotal role in industrial chemistry, particularly in the synthesis of esters and ethers. They are also crucial in the sol-gel process for producing metal oxides, used in coatings, ceramics, and advanced materials. Their reactivity makes them suitable catalysts and intermediates in various chemical processes, including the production of biodiesel through transesterification.
Phenoxides in Synthesis and Manufacturing
Phenoxides find their utility in the synthesis of aryl ethers, serving as intermediates in the production of dyes, resins, and plastics. Their stability, coupled with selective reactivity, makes them valuable in pharmaceutical synthesis, where they act as key intermediates in constructing complex organic molecules.
Medicinal Chemistry
Role of Alkoxides and Phenoxides in Drug Development
Both alkoxides and phenoxides are instrumental in medicinal chemistry, aiding in the synthesis of active pharmaceutical ingredients (APIs). Their reactivity profiles allow for the selective formation of ether and ester bonds, crucial in the molecular architecture of many drugs.
Example Compounds
- Aspirin, one of the most well-known drugs, involves the use of phenoxide ions in its synthesis, showcasing the role of phenoxides in creating medically important compounds.
- Sildenafil (Viagra), a treatment for erectile dysfunction, utilizes alkoxide chemistry in one of its key synthesis steps, highlighting the importance of alkoxides in drug development.
Frequently Asked Questions
What are alkoxides?
Alkoxides are organic compounds characterized by an alkoxide ion, where a metal atom is bonded to an oxygen atom derived from an alcohol. These compounds are highly reactive, primarily used in organic synthesis and as catalysts in various chemical reactions. Their reactivity and utility are influenced by the nature of the metal and the alkyl group attached to the oxygen atom.
How do phenoxides differ from alkoxides?
Phenoxides differ from alkoxides in their structure and origin; they are derived from phenols rather than aliphatic alcohols. This difference leads to distinct chemical properties, notably phenoxides exhibit enhanced stability due to the resonance stabilization provided by the aromatic ring. This stability impacts their reactivity, making them valuable in certain chemical syntheses, particularly in the formation of ethers and as intermediates in various organic reactions.
What are the applications of alkoxides and phenoxides?
Alkoxides and phenoxides find extensive applications in both academic and industrial chemistry. Alkoxides are crucial in the synthesis of esters, ethers, and other compounds, serving as intermediates in various chemical processes. Phenoxides, on the other hand, are used in the production of aromatic ethers, as catalysts in organic reactions, and in the manufacture of dyes, drugs, and polymers. Their unique properties make them indispensable in the development of new materials and pharmaceuticals.
How does the structure of alkoxides and phenoxides affect their reactivity?
The structure of alkoxides and phenoxides directly influences their reactivity. Alkoxides, with their aliphatic structure, tend to be more reactive towards nucleophilic substitution and elimination reactions. Phenoxides, enriched by the aromatic ring, show stability against such reactions due to resonance effects. This structural distinction dictates their suitability and efficiency in specific chemical transformations and applications.
Conclusion
Alkoxides and phenoxides embody the diversity and complexity of organic chemistry, illustrating how structural nuances can profoundly affect chemical properties and reactivity. The exploration of these compounds not only enriches our understanding of organic synthesis but also highlights the importance of molecular architecture in determining the behavior of chemical species. Their varied applications, from the synthesis of complex molecules to their roles in industrial processes, underscore the practical significance of these compounds in scientific and technological advancements.
The knowledge of differences between alkoxides and phenoxides is not merely academic; it serves as a foundation for innovation in chemical synthesis, environmental sustainability, and pharmaceutical development. As chemistry continues to evolve, the study of such compounds will remain at the forefront of scientific inquiry, driving progress in myriad fields and contributing to solutions for global challenges.