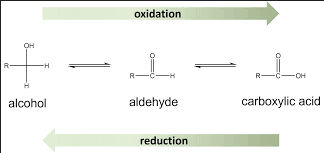
Organic chemistry is a vast field that deals with carbon-containing compounds, among which aldehydes and alcohols are fundamental. Each group plays a critical role in both natural processes and synthetic applications. Despite their similar molecular structures, these compounds exhibit distinct properties and uses. This segment aims to clarify the differences between aldehydes and alcohols, highlighting their unique characteristics and importance.
Aldehydes are organic compounds characterized by a carbonyl group (a carbon atom double bonded to an oxygen atom) connected to at least one hydrogen atom, represented as -CHO. Alcohols, on the other hand, contain a hydroxyl group (-OH) bonded to a carbon atom. The presence of these functional groups imparts different physical and chemical properties to aldehydes and alcohols, which affects their reactivity and applications.
Aldehydes are typically more reactive than alcohols due to their carbonyl group, making them crucial in organic synthesis and industrial processes. Alcohols, being less reactive, find extensive use as solvents, in beverages, and in the production of other chemicals. Understanding these differences is essential for professionals working with these compounds in various industries.

Basic Concepts
Definition of Aldehydes
Aldehydes are a class of organic compounds prominently known for their carbonyl group (C=O) attached to at least one hydrogen atom. This structural feature is the cornerstone of their chemical identity. The general formula for an aldehyde is RCHO, where R represents any alkyl or aryl group. Aldehydes are ubiquitous in many biological processes and are commonly used in the synthesis of other chemicals. They are typically formed by the partial oxidation of alcohols and are more reactive due to the presence of the carbonyl group.
Definition of Alcohols
Alcohols are organic compounds characterized by the presence of one or more hydroxyl groups (-OH) attached to a carbon atom. Their general formula is R-OH, where R can be any alkyl or aryl group. Alcohols are versatile compounds found in various applications from industrial solvents and beverages to fuels and antiseptics. They can be produced through several methods, including fermentation and hydration of alkenes.
Chemical Structures
Structure of Aldehydes
The structural framework of aldehydes includes a carbon atom double bonded to an oxygen atom to form a carbonyl group, with one of the bonds to this carbon coming from a hydrogen atom. This simple yet reactive group makes aldehydes particularly interesting in organic chemistry. The presence of the hydrogen atom distinguishes aldehydes from ketones, which have two alkyl groups attached to the carbonyl carbon.
Structure of Alcohols
The defining feature of alcohols is their hydroxyl group. This group consists of an oxygen atom bonded to a hydrogen atom, attached directly to the carbon skeleton of the organic molecule. The carbon bonded to the OH group can be part of a simple alkyl chain or a more complex ring structure, affecting the properties and reactivity of the alcohol.

Physical Properties
Boiling and Melting Points
Aldehydes and alcohols show significant differences in their boiling and melting points, primarily due to their differing polarity and the ability to form hydrogen bonds. Generally, alcohols have higher boiling points than aldehydes of similar molecular weight because the hydroxyl group in alcohols leads to strong intermolecular hydrogen bonding. Melting points also vary, with alcohols usually having higher values compared to aldehydes, which can often be attributed to their structured hydrogen bonding networks.
Solubility Differences
Solubility in water is another area where aldehydes and alcohols differ significantly. Alcohols are typically more soluble in water than aldehydes due to their ability to form hydrogen bonds with water molecules. This property makes alcohols useful as solvents and in industries where water solubility is crucial. In contrast, the solubility of aldehydes decreases as the length of the carbon chain increases, due to the increasing nonpolar character of the alkyl group.
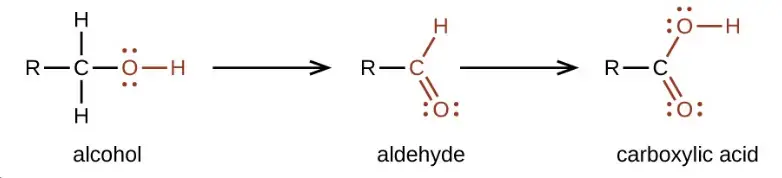
Chemical Reactions
Reactions Specific to Aldehydes
Aldehydes are known for their reactivity, particularly in nucleophilic addition reactions due to the electrophilic nature of the carbonyl carbon. Common reactions include:
- Cannizzaro reaction: where a non-enolizable aldehyde is treated with a strong base, resulting in disproportionation into an alcohol and a carboxylic acid.
- Addition of Grignard reagents: leading to the formation of secondary and tertiary alcohols. These reactions are critical in the synthesis of various organic compounds and pharmaceuticals.
Reactions Specific to Alcohols
Alcohols participate in a wide range of reactions, reflecting their versatility:
- Dehydration to alkenes: under acidic conditions, alcohols lose water molecules to form double bonds.
- Esterification: where alcohols react with carboxylic acids to form esters, a reaction used in the production of flavors, fragrances, and polymers. These reactions exploit the reactivity of the hydroxyl group and are foundational in organic synthesis.
Comparison of Reactivity
When comparing reactivity, aldehydes generally exhibit greater reactivity than alcohols due to the carbonyl group’s electrophilic character. This makes aldehydes more susceptible to nucleophilic attacks, whereas alcohols, being less electrophilic, are more resistant to such reactions. This fundamental difference is key to their distinct roles in chemical syntheses and applications.
Production Processes
How Aldehydes are Produced
Aldehydes are commonly produced through several methods, depending on the type and required purity of the aldehyde. The primary methods include:
- Oxidation of Alcohols: Aldehydes can be synthesized by the partial oxidation of primary alcohols. Catalysts such as silver or copper are often used to facilitate this reaction.
- Oxidative Dehydrogenation: In this method, hydrocarbons are partially oxidized to form aldehydes, using catalysts like molybdenum.
- Hydroformylation: Also known as the oxo process, involves the reaction of alkenes with carbon monoxide and hydrogen, producing aldehydes as intermediates in the synthesis of alcohols.
These processes highlight the versatility of aldehyde production, tailored to meet industrial demands and specifications.
How Alcohols are Produced
Alcohols are produced through various processes including:
- Fermentation: This biological process converts sugars into alcohol and carbon dioxide via yeast. It is the traditional method for producing ethanol.
- Hydration of Alkenes: Industrial production of alcohols such as ethanol and propanol often involves the hydration of alkenes in the presence of acid catalysts.
- Reduction of Aldehydes and Ketones: Alcohols can also be produced by reducing aldehydes and ketones using reducing agents like lithium aluminium hydride.
These methods underscore the diverse industrial applications of alcohols, ranging from beverages to biofuels.
Uses and Applications
Industrial Uses of Aldehydes
Aldehydes serve a crucial role in various industries due to their reactivity:
- Pharmaceuticals: Synthesis of drugs such as antidepressants and antipyretics.
- Perfumes and Flavors: Aldehydes contribute to the synthesis of aromatic compounds used in perfumery and flavoring agents.
- Resins and Plastics: Formaldehyde, a simple aldehyde, is used in the production of resins, adhesives, and coatings.
Industrial Uses of Alcohols
Alcohols are integral to numerous sectors:
- Solvents: Due to their ability to dissolve both organic and inorganic substances.
- Fuel: Ethanol is widely used as a biofuel.
- Consumer Goods: Alcohols are used in the manufacture of household cleaners, disinfectants, and cosmetics.
Health and Safety
Toxicity of Aldehydes
Aldehydes, especially formaldehyde, are known for their toxicity and potential health risks:
- Respiratory Issues: Inhalation can lead to respiratory irritation and long-term health problems.
- Carcinogenic Properties: Certain aldehydes have been linked to cancer, necessitating stringent handling protocols.
Toxicity of Alcohols
While alcohols are generally less toxic than aldehydes, they still pose risks:
- Ethanol Toxicity: Consumption can lead to alcohol poisoning and long-term health issues.
- Methanol: Extremely toxic and can cause severe health effects including blindness and death.
Safety Measures
Due to these hazards, robust safety measures are essential:
- Ventilation: Proper ventilation in areas where aldehydes and alcohols are used or produced.
- Personal Protective Equipment (PPE): Use of gloves, goggles, and respirators.
- Training: Comprehensive training for all personnel handling these chemicals.
Detection and Identification
Laboratory Detection of Aldehydes
Detecting aldehydes involves specific chemical tests:
- Schiff’s Test: A reagent that turns magenta in the presence of aldehydes.
- Tollens’ Test: Silver mirror test used to confirm the presence of aldehydes.
Laboratory Detection of Alcohols
Detection methods for alcohols include:
- Chromic Acid Test: Changes color in the presence of primary and secondary alcohols.
- Lucas Test: Differentiates alcohols based on reactivity with zinc chloride.
Environmental Impact
Environmental Risks of Aldehydes
Aldehydes, particularly formaldehyde, pose significant environmental risks:
- Air Quality Degradation: Volatile organic compounds (VOCs) from aldehydes contribute to pollution and smog.
- Water Contamination: Improper disposal can lead to water contamination.
Environmental Risks of Alcohols
Alcohols affect the environment differently:
- Bioaccumulation: Some alcohols can accumulate in the ecosystem, posing risks to wildlife and plants.
- Oxygen Demand: High levels of alcohols in water bodies can deplete oxygen, affecting aquatic life.
Sustainable Alternatives
In response to these impacts, research into sustainable alternatives is vital:
- Green Chemistry: Developing less toxic compounds and processes.
- Recycling and Reuse: Systems for recycling alcohols and aldehydes to minimize environmental footprint.
Frequently Asked Questions
What are aldehydes?
Aldehydes are a type of organic compound characterized by the presence of a carbonyl group bonded to a hydrogen atom and one other R group (which can be a hydrogen or carbon-containing group). This structure makes them highly reactive and useful in a variety of chemical syntheses.
How do alcohols differ from aldehydes?
Alcohols differ from aldehydes primarily in their functional groups. Alcohols contain a hydroxyl group (-OH), making them polar and capable of forming hydrogen bonds. This gives alcohols distinct properties like higher boiling points and the ability to dissolve in water and other polar substances.
Why are aldehydes more reactive than alcohols?
Aldehydes are more reactive than alcohols due to the presence of the carbonyl group, which is more electrophilic. This makes aldehydes susceptible to nucleophilic attacks, a common mechanism in many organic reactions, unlike the more stable hydroxyl group of alcohols.
What are common uses of aldehydes and alcohols?
Aldehydes are commonly used in the manufacture of perfumes, plastics, and dyes due to their ability to undergo a variety of chemical reactions. Alcohols are widely used as solvents, in the production of beverages, and as intermediates in the synthesis of other chemicals.
How are aldehydes and alcohols detected?
Both aldehydes and alcohols can be detected and quantified by specific chemical tests that exploit their unique functional groups. Aldehydes are typically identified using the Schiff test, which turns pink in their presence. Alcohols are often tested using the Lucas reagent, which reacts differently depending on the alcohol’s structure.
Conclusion
In conclusion, while aldehydes and alcohols share some structural similarities, their chemical properties and applications differ significantly. Aldehydes’ reactivity makes them indispensable in synthetic chemistry, whereas alcohols’ versatility sees them used widely across various industries. Recognizing these differences is crucial for anyone involved in the production or use of these chemicals.
This exploration not only distinguishes aldehydes from alcohols but also emphasizes the importance of understanding their distinct characteristics in scientific and industrial contexts. This knowledge can aid in the development of new technologies and improve existing processes in fields ranging from pharmaceuticals to environmental science.