Calorimetry, the science of measuring heat changes in chemical reactions or physical changes, stands as a cornerstone in the realms of chemistry, physics, and biology. Its application spans from determining nutritional content in food to exploring the energy dynamics of chemical reactions. Among the various types of calorimeters, the adiabatic and isoperibol calorimeters are particularly noteworthy for their distinct methods of measuring heat exchange, each suited to specific scientific inquiries.
The adiabatic calorimeter operates by ensuring that no heat is exchanged with its surroundings, focusing on the system’s internal heat changes. In contrast, the isoperibol calorimeter maintains the surrounding temperature constant, measuring the heat exchanged between the system and its environment. These differences underscore their unique applicability and accuracy in experimental settings, making them invaluable tools in scientific research.
Understanding these devices’ operational principles, features, and applications highlights the nuanced approach scientists take towards measuring thermal changes. It reveals the meticulous care with which experiments must be designed to yield accurate, reliable data, emphasizing the importance of selecting the right calorimeter based on the specific research requirements and conditions under which a study is conducted.
Calorimetry Basics
Definition and Purpose
Calorimetry is the scientific measurement of heat exchange in chemical reactions or physical changes. At its core, calorimetry allows scientists to understand the energy dynamics of these processes, providing insights into reaction energetics, material properties, and thermodynamic principles. The fundamental purpose of this technique is to quantify the energy changes involved, which are critical for applications ranging from food nutrition analysis to new material development.
Key Components of a Calorimeter
A typical calorimeter consists of several key components:
- Reaction Chamber: The space where the chemical reaction or physical change occurs.
- Temperature Measurement System: Devices like thermocouples or thermistors that measure temperature changes.
- Insulation: Materials that minimize heat exchange with the environment, ensuring accurate measurements.
- Stirring Mechanism: Ensures uniform temperature distribution within the chamber.
- Calibration System: Ensures the accuracy of temperature measurements and heat calculations.
These components work together to accurately track the heat involved in the system under study.
The Role of Heat Exchange
Heat exchange is central to calorimetry, as it is the measurement of this exchange that allows for the determination of enthalpy changes in reactions or processes. Whether heat is absorbed or released during a process indicates the endothermic or exothermic nature of the reaction, respectively. Understanding this heat exchange is crucial for predicting reaction outcomes, designing chemical processes, and exploring material properties.
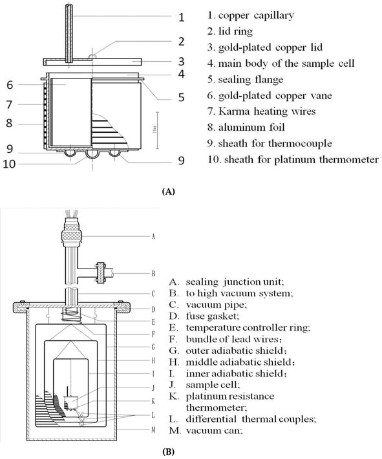
Adiabatic Calorimeter
Definition and Principles
An adiabatic calorimeter operates on the principle of zero heat exchange with its surroundings. It ensures that all the heat generated or absorbed in a reaction remains within the system, allowing for precise measurement of internal energy changes. This method is especially useful for highly reactive or explosive materials, where controlling external heat exchange is crucial for safety and accuracy.
How It Works
The adiabatic process involves:
- Sealing the sample in the reaction chamber.
- Minimizing external heat exchanges through insulation.
- Monitoring the temperature change that occurs solely due to the reaction.
This setup provides a direct measurement of the reaction’s internal energy change, as no external heat influences the system.
Key Features
- High Precision: By eliminating external heat exchange, it offers high accuracy in measuring heat changes.
- Safety: Ideal for studying reactive substances by preventing heat loss or gain that could influence the reaction.
- Control: Allows precise control over reaction conditions, essential for sensitive experiments.
Applications
Adiabatic calorimeters are vital in sectors where understanding the energy dynamics of reactions under isolated conditions is crucial. This includes:
- Safety Testing: For explosives and reactive chemicals, determining the conditions under which materials might react dangerously.
- Thermodynamics Research: Studying the fundamental energy changes in chemical reactions.
- Material Science: Evaluating the heat capacity and energy transitions in new materials.
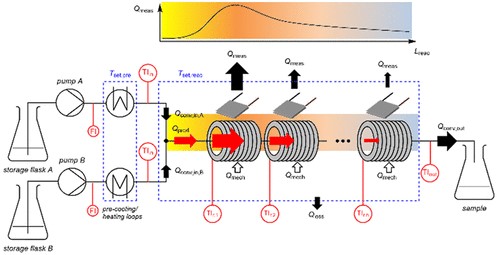
Isoperibol Calorimeter
Definition and Principles
In contrast to the adiabatic approach, the isoperibol calorimeter maintains a constant temperature of its surroundings, focusing on the heat exchanged between the system and its environment. This method is particularly suited for reactions where maintaining a controlled external temperature provides insight into the system’s thermal behavior under steady-state conditions.
Operation Mechanism
The isoperibol calorimeter operates by:
- Encasing the reaction chamber within an environment whose temperature is meticulously controlled and kept constant.
- Measuring the heat that flows between the reaction chamber and its surroundings, as the system seeks equilibrium.
- Calculating the heat exchange based on the temperature difference and the known thermal properties of the system.
This setup allows for accurate measurement of heat exchange, crucial for understanding processes that interact with their surroundings.
Unique Characteristics
- Steady-State Measurement: Ideal for long-term observations of slow reactions.
- Environmental Interaction: Allows the study of how systems exchange heat with their environment, critical for many biological and environmental processes.
- Versatility: Suitable for a wide range of temperatures and reaction conditions.
Uses in Research
Isoperibol calorimeters find their applications in areas requiring an understanding of heat exchange with the environment, including:
- Environmental Science: Studying soil or atmospheric processes where temperature regulation is essential.
- Biological Research: Measuring metabolic rates and energy expenditure in organisms.
- Chemical Engineering: Designing processes that rely on precise temperature conditions for optimal efficiency.
Comparative Analysis
Design Differences
Construction and Materials
The construction and materials used in adiabatic and isoperibol calorimeters significantly influence their functionality and application. Adiabatic calorimeters are designed with high-grade insulation materials to prevent any heat exchange with the environment. Materials such as vacuum panels and reflective coatings are common to minimize heat transfer. On the other hand, isoperibol calorimeters focus on maintaining a constant temperature environment, utilizing materials that facilitate precise temperature control, such as water jackets or electronically controlled heating/cooling systems.
Control Systems
The control systems of these calorimeters also differ markedly. Adiabatic calorimeters employ systems that monitor and adjust the internal temperature without external influence, relying on sensitive temperature sensors and feedback mechanisms. Isoperibol calorimeters, conversely, use control systems designed to maintain the external temperature at a steady state, often requiring more complex external temperature regulation mechanisms.
Heat Exchange
Adiabatic Process
In the adiabatic process, heat exchange with the surroundings is nullified, ensuring that any temperature change is due to the internal reaction alone. This isolation is key to studying exothermic and endothermic reactions where external thermal influences must be eliminated to obtain accurate data.
Isoperibol Conditions
Under isoperibol conditions, the calorimeter measures how the system exchanges heat with its controlled environment. This method is essential for understanding reactions that naturally interact with their surroundings, providing insights into real-world process dynamics and energy flow.
Accuracy and Sensitivity
Factors Affecting Measurements
Several factors can impact the accuracy and sensitivity of calorimetric measurements, including:
- Calibration: Regular calibration is essential for maintaining measurement accuracy.
- Sample Size and Composition: Variations can affect heat capacity and thermal conductivity.
- Environmental Conditions: External temperature and pressure changes can influence results.
Comparative Precision
Adiabatic calorimeters typically offer higher precision in measuring heat changes due to their isolated systems. Isoperibol calorimeters, while precise within their operational parameters, may be more susceptible to environmental fluctuations, impacting their sensitivity in certain applications.
Application Spectrum
Suitable Experiments for Each
Adiabatic calorimeters are best suited for experiments requiring precise measurement of heat changes without external interference, such as in the study of explosive materials or fast reactions. Isoperibol calorimeters are ideal for long-duration studies or those involving slow biochemical processes, where the interaction with a stable external temperature is crucial.
Industry-specific Uses
In the pharmaceutical industry, adiabatic calorimeters can help in the safety assessment of new compounds. Isoperibol calorimeters find use in food science, measuring caloric content under consistent environmental conditions. Both types have broad applications across chemical engineering, environmental science, and materials research, tailored to specific experimental needs.
Maintenance and Calibration
Routine Procedures
Regular maintenance and calibration are vital to ensure the long-term accuracy and reliability of calorimeters:
- Cleaning: Keeping the reaction chamber and sensors clean prevents measurement errors.
- Calibration Checks: Frequent calibration with standard materials ensures consistent accuracy.
- Software Updates: Updating control software can improve functionality and data analysis capabilities.
Impact on Long-term Accuracy
Adhering to these procedures minimizes drift in measurements and extends the equipment’s operational life, maintaining the integrity of research results over time.
Choosing the Right Calorimeter
Factors to Consider
Selecting the appropriate calorimeter involves considering:
- Research Objectives: The type of reactions or processes to be studied.
- Precision Requirements: The level of accuracy needed for the experiments.
- Budget and Resources: Initial costs and ongoing maintenance requirements.
Matching Technology to Research Needs
Understanding the unique capabilities and limitations of adiabatic and isoperibol calorimeters allows researchers to match the technology to their specific research needs, optimizing experimental outcomes and advancing scientific understanding.
Future of Calorimetry
Technological Advancements
Emerging technologies in material science and electronic control systems are set to enhance the precision, usability, and application range of calorimeters. Innovations such as nanomaterial-based insulation and AI-driven temperature control can revolutionize how calorimetry is performed, offering even greater insights into thermal phenomena.
Emerging Trends
The integration of calorimeters with other analytical techniques and the development of portable, field-use models are among the trends shaping the future of calorimetry. These advancements promise to expand the scope of calorimetric analysis, bringing it into new areas of research and application, from on-site environmental monitoring to in-field biological studies.
Frequently Asked Questions
What is calorimetry?
Calorimetry is a branch of science focused on measuring the heat of chemical reactions or physical changes. This measurement is crucial for understanding the energy dynamics of various processes, essential in fields such as chemistry, physics, and biology.
How do adiabatic and isoperibol calorimeters differ?
Adiabatic and isoperibol calorimeters differ primarily in their approach to heat exchange. Adiabatic calorimeters prevent heat exchange with the environment, focusing on internal heat changes. In contrast, isoperibol calorimeters keep the surrounding temperature constant, measuring heat exchange between the system and its environment.
Why is choosing the right calorimeter important in scientific research?
Selecting the appropriate calorimeter for scientific research is critical due to the precision it brings to thermal measurements. The choice between an adiabatic and isoperibol calorimeter affects the accuracy, sensitivity, and applicability of the results, influencing the overall validity of the research findings.
Can calorimeters be used in industry applications?
Yes, calorimeters have significant applications in various industries. They are used to measure the energy content of foods, materials’ combustion efficiency, and many other processes where energy exchange is a key factor. The choice between adiabatic and isoperibol calorimeters depends on the specific requirements of the application.
Conclusion
In the exploration of heat changes within chemical and physical processes, the distinction between adiabatic and isoperibol calorimeters embodies the precision and adaptability of modern scientific inquiry. These instruments, each with its methodological strengths and areas of application, offer researchers a nuanced understanding of energy dynamics. Their selection is not merely a technical decision but a foundational one that shapes the course and credibility of scientific investigations.
Embracing these tools’ capabilities and understanding their differences allows scientists to push the boundaries of what we know about the thermal aspects of matter. As technology evolves, so too will the precision and application of calorimeters, continuing to illuminate the unseen processes that drive the natural and designed world around us.

