Acrylates and methacrylates are fundamental components widely utilized in various industrial applications, ranging from simple household products to complex automotive parts. Each compound offers unique properties and benefits, making them indispensable in modern manufacturing processes. Though they share similar names and chemical bases, the differences between them impact their usability and effectiveness in different scenarios.
The primary difference between acrylate and methacrylate lies in their chemical structures. Acrylates are known for their excellent weathering properties and flexibility, while methacrylates boast greater hardness and heat resistance. These distinctions not only influence how each compound is used but also affect their production processes and physical characteristics.
In the realms of industry and science, acrylates and methacrylates serve as critical materials in the creation of polymers and plastics. Their varied applications underscore the importance of understanding each compound’s specific attributes and environmental impacts. This knowledge ensures appropriate and safe use in both existing and emerging technologies.
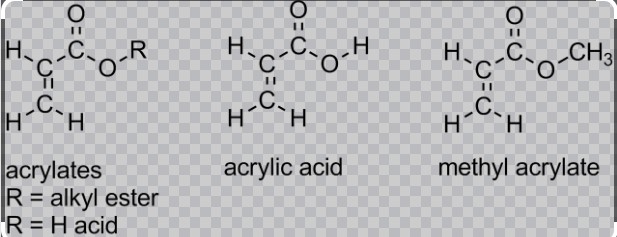
Chemical Structures
Understanding the basic chemical structures of acrylate and methacrylate is essential for comprehending their diverse applications and properties. These structures serve as the foundation for their physical and chemical characteristics, which in turn influence their suitability for various industrial uses.
Basic Structure of Acrylate
Acrylates, or polyacrylates, are derived from acrylate monomers, which consist of a vinyl group linked to a carboxylic acid ester group. The general formula for these monomers is CH2=CHCOOR, where R represents an alkyl group that can vary in length and branching. This variation in the R group allows for a range of polymers with different properties to be synthesized, tailoring materials to specific needs.
Basic Structure of Methacrylate
Methacrylates, similar to acrylates, are based on the methacrylic acid. The structural formula for methacrylate monomers is CH2=C(CH3)COOR, where the presence of a methyl group attached to the double-bonded carbon distinguishes it from acrylate. This methyl group impacts the polymer’s properties, such as increasing the glass transition temperature, which affects the material’s heat resistance.
Comparison of Structures
Comparing acrylate and methacrylate structures reveals key differences:
- Acrylates lack the methyl group present in methacrylates, which results in softer and more flexible polymers.
- Methacrylates have higher thermal stability and strength due to the methyl group, making them suitable for more rigid applications.
Production Processes
The production processes for acrylate and methacrylate not only highlight the complexity of chemical engineering but also reflect the adaptability of these compounds to meet various industrial requirements.
How Acrylate Is Made
The production of acrylate typically involves the following steps:
- Raw Material Preparation: Acrylic acid is produced primarily from the oxidation of propylene, a byproduct of fossil fuel refining.
- Esterification: Acrylic acid is then esterified with an alcohol, such as methanol or ethanol, in the presence of a catalyst to form the acrylate ester.
- Purification: The ester is purified through distillation to achieve the desired quality and specifications for various applications.
How Methacrylate Is Made
Methacrylate production mirrors that of acrylate but with some distinctions:
- Raw Material Preparation: Methacrylic acid is synthesized from acetone and cyanohydrin, derived from propylene.
- Esterification: Similar to acrylate, methacrylic acid is esterified with an alcohol, but the process often involves different catalysts or conditions to accommodate the methyl group.
- Purification: Distillation is employed to purify the methacrylate ester, ensuring it meets industry standards.
Comparative Analysis
While both processes involve similar steps, the choice of raw materials and specific conditions used during synthesis and purification differ, influenced by the unique properties required of the final product.
Properties and Characteristics
The properties of acrylates and methacrylates determine their functionality in a broad array of products and applications.
Physical Properties
Melting and Boiling Points:
- Acrylates generally have lower melting and boiling points due to their less rigid molecular structure.
- Methacrylates, with their additional methyl group, exhibit higher melting and boiling points, correlating with their increased thermal stability.
Solubility and Viscosity:
- Acrylates are typically more soluble in water and various organic solvents than methacrylates. This property makes acrylates preferable for applications requiring good water dispersibility.
- The viscosity of acrylates is lower, which facilitates easier processing and application in formulations like paints and coatings.
Chemical Properties
Reactivity:
- Both acrylates and methacrylates are highly reactive with a variety of co-reactants, which allows them to polymerize readily. Methacrylates, however, are slightly less reactive due to steric hindrance from the methyl group.
Stability:
- Acrylates are less stable compared to methacrylates, especially under UV light and heat, leading to quicker degradation. This aspect is critical in applications requiring long-term durability.
- Methacrylates offer enhanced stability, making them suitable for products exposed to harsher conditions.
Applications in Industry
Acrylate and methacrylate compounds are integral to a multitude of industrial applications. Their unique properties make them essential in the manufacture of a variety of products ranging from everyday items to specialized applications.
Acrylate Uses
Acrylates are versatile materials used extensively across different sectors. Their ability to quickly form films and adhere to substrates makes them ideal for numerous applications.
Paints and Coatings
Acrylates are predominantly used in the production of paints and coatings due to their excellent adhesion and glossy finish. These coatings are applied in:
- Automotive finishes that require a durable, UV-resistant layer.
- Protective coatings for buildings and structures, offering resistance against weather and corrosion.
- Decorative finishes in homes and on furniture, providing aesthetic appeal along with protection.
Adhesives and Sealants
In the realm of adhesives and sealants, acrylates provide strong bonding properties. They are crucial in:
- Packaging industries, where quick-setting and strong adhesion are necessary.
- Construction, for sealing joints and cracks, ensuring structural integrity and durability.
- Medical adhesives, used in bandages and surgical tapes, where non-toxic and skin-friendly properties are essential.
Methacrylate Uses
Methacrylates, with their enhanced strength and thermal stability, serve critical roles in more demanding environments.
Automotive Parts
Methacrylates are key in manufacturing automotive parts, where high strength and resistance to wear are required. Uses include:
- External panels and trims that require paint that adheres well and withstands environmental stress.
- Internal components like dashboards and light covers, which benefit from methacrylate’s clarity and durability.
Plexiglass and Other Plastics
Methacrylates are also crucial in producing plexiglass and various other plastics, valued for their clarity and impact resistance. Applications include:
- Windows and shields in vehicles and buildings, providing safety and insulation.
- Displays and signs, where transparency and durability are paramount.
Health and Environmental Impact
The production and use of acrylates and methacrylates have significant implications for health and the environment, necessitating stringent safety measures and environmental considerations.
Toxicity and Safety Measures
Handling acrylates and methacrylates requires careful attention due to their toxicity. Safety measures include:
- Use of protective gear to prevent skin contact and inhalation.
- Adequate ventilation in workplaces to mitigate fume inhalation.
- Training for workers on handling emergencies involving these chemicals.
Environmental Concerns
Biodegradability
The environmental impact of acrylates and methacrylates is a concern, particularly in terms of biodegradability. These compounds do not easily break down, leading to potential long-term effects on ecosystems if not managed properly.
Impact on Wildlife
Both acrylates and methacrylates can have adverse effects on wildlife, particularly aquatic life, due to their persistence in the environment. Measures to mitigate this impact include:
- Development of less toxic derivatives.
- Improved waste management practices to prevent chemical runoff into natural habitats.
Market Trends
The global market for acrylates and methacrylates continues to grow, driven by demand in various industries and emerging markets.
Global Demand and Supply
Current trends show increasing demand for these compounds in developing regions, coupled with innovations in production technology that aim to meet this demand sustainably. Supply chain optimizations are also in focus to ensure steady availability of raw materials and finished products.
Innovations and Future Prospects
Innovation in the field of acrylates and methacrylates is geared towards enhancing their properties and reducing their environmental footprint. Prospects include:
- Developing new formulations that are less harmful and more biodegradable.
- Advancements in recycling technologies to reclaim and reuse these materials in various applications.
Frequently Asked Questions
What are acrylates used for?
Acrylates are primarily used in the production of paints, coatings, adhesives, and textiles. They provide enhanced durability and flexibility, making them ideal for outdoor applications and items requiring a long-lasting finish.
How are methacrylates different from acrylates?
Methacrylates differ from acrylates mainly in their resistance to heat and impact. This makes methacrylates suitable for use in more demanding environments, such as in automotive parts and safety glass.
Are acrylates and methacrylates environmentally friendly?
Both compounds raise environmental concerns, particularly in terms of biodegradability and potential toxicity. However, ongoing research aims to improve their formulations to reduce their environmental footprint and enhance safety measures during production and disposal.
Can acrylates and methacrylates be recycled?
Yes, products made from acrylates and methacrylates can often be recycled, depending on their chemical formulation and the recycling facilities available. Recycling helps mitigate their environmental impact and promotes sustainable use of resources.
Conclusion
The distinction between acrylate and methacrylate is crucial for various industrial applications, influencing decisions from production to final product design. As industries continue to evolve, the specific properties of each compound will play a significant role in shaping innovations and improving sustainability practices.
In conclusion, while both acrylates and methacrylates are integral to modern manufacturing, ongoing research and development are essential to maximizing their benefits while minimizing their environmental impacts. Understanding their unique characteristics enables more informed choices in their application, driving forward both technological advancement and environmental responsibility.