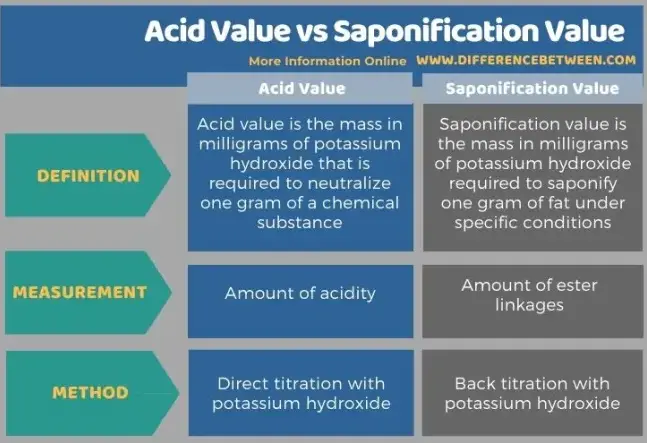
Acid and saponification values are critical metrics used in various industries to assess the quality and chemical properties of fats and oils. These values are particularly significant in the realms of food production, cosmetics, and biofuels, where they help manufacturers ensure product consistency and effectiveness. Each value measures a different aspect of the oil or fat, offering insights into its composition and potential uses.
The acid value measures the free fatty acid content of an oil or fat, indicating its purity and degree of degradation. In contrast, the saponification value quantifies the total fatty acids present, providing a measure of the potential yield of soap from a given fat or oil. Understanding these values helps in assessing the suitability of a fat or oil for specific applications and in monitoring quality during production.
While both values serve as benchmarks for the chemical properties of oils and fats, they differ fundamentally in their measurement and implications. The acid value is a direct indicator of the freshness and deterioration of oils, vital for quality control in edible oils. The saponification value, meanwhile, is essential for determining the economic and practical feasibility of using a specific oil for soap making or in biodiesel production.
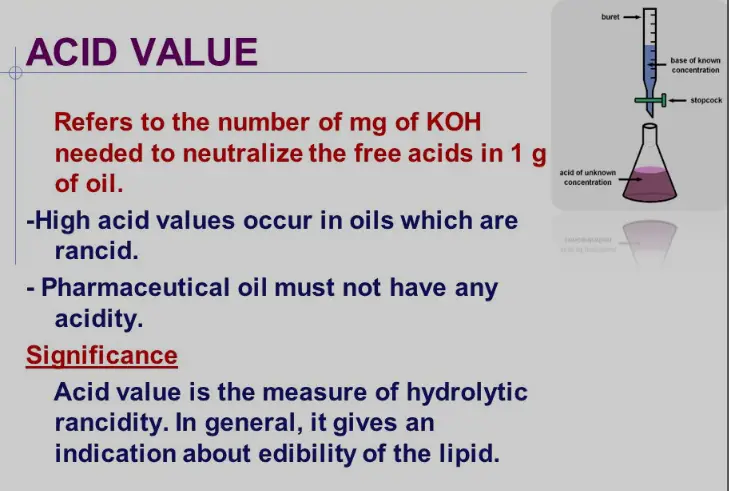
Acid Value Explained
Definition
The Acid Value is a crucial parameter in assessing the quality of fats and oils. It quantifies the amount of free fatty acids present in a substance, measured in milligrams of potassium hydroxide (KOH) required to neutralize the free acids in one gram of fat. Typically, a lower acid value indicates fresher oil, as it signifies minimal oxidative degradation.
Measurement Methods
To accurately determine the acid value, several steps are employed:
- Preparation of Sample: The oil or fat is prepared by ensuring it is clear, free of impurities, and at a consistent temperature.
- Titration Setup: A known quantity of the sample is dissolved in a solvent mix, usually involving alcohol and an indicator like phenolphthalein, which changes color in the presence of a base.
- Titration Process: The solution is titrated with a standardized solution of KOH until the endpoint, which is the point at which the color changes, indicating that all free fatty acids have been neutralized.
- Calculation: The volume of KOH used is noted, and the acid value is calculated using the formula:Acid Value=Volume of KOH×Normality of KOH×56.1Weight of SampleAcid Value=Weight of SampleVolume of KOH×Normality of KOH×56.1
Saponification Value Defined
Definition
The Saponification Value measures the total fatty acids present in fats and oils, indicating how much KOH (in mg) is needed to saponify one gram of fat. It reflects the molecular weight of the triglycerides within the oil, helping gauge its potential uses in products like soaps or biodiesel.
Calculation Process
The calculation of the saponification value involves:
- Weighing the Sample: A precise amount of fat or oil is weighed.
- Reaction Setup: The sample is mixed with a known excess of alcoholic KOH solution and heated to ensure complete saponification.
- Back Titration: After the reaction, the excess KOH is titrated with a standard solution of hydrochloric acid.
- Final Calculation: The saponification value is calculated by noting the amount of KOH that reacted with the fat. The formula used is:Saponification Value=(Volume of HCl used in titration)×Normality of HCl×56.1Weight of the SampleSaponification Value=Weight of the Sample(Volume of HCl used in titration)×Normality of HCl×56.1
Key Differences
Chemical Basis
The chemical basis of the acid and saponification values lies in their focus: acid value measures free fatty acids resulting from the breakdown of triglycerides, whereas the saponification value measures the total triglycerides present, indicating potential yield for products requiring fatty acids.
Practical Implications
The practical implications of these values are vast. For example, a high acid value often suggests poor quality oil, prone to spoilage, affecting its suitability for consumption or certain industrial uses. Conversely, a high saponification value typically indicates a higher potential for soap production, crucial in manufacturing sectors.
Measurement Techniques
Tools and Techniques for Acid Value
To measure the acid value effectively:
- Digital Titrators: These offer precision in adding the titrant, improving the accuracy of the endpoint detection.
- Color Indicators: These are essential for visually determining the titration endpoint.
- Thermometers and Heaters: Consistent sample temperature is crucial for accurate measurements.
Tools and Techniques for Saponification Value
For saponification value measurement:
- Reflex Condensers: Used to prevent the evaporation of the alcoholic KOH solution during the reaction.
- Burette and Pipette: Ensure precise measurement and delivery of reagents.
- Balances: Accurate weighing of the sample is critical for reliable results.

Industrial Applications
Uses in Food Industry
The acid and saponification values are pivotal in the food industry, where the quality and safety of edible fats and oils are paramount. The acid value, in particular, serves as a critical indicator of oil quality and freshness. It helps manufacturers determine whether the oil is suitable for consumption or needs refining. Lower acid values are preferred for cooking oils because they indicate less oxidation and spoilage.
The saponification value also has its applications in the food sector, primarily in the area of ingredient analysis and food science research. It provides essential data on the molecular weight and composition of fats used in products, influencing decisions about shelf life and storage conditions.
- Quality Control: Regular testing of acid values helps in maintaining the quality of oils in production.
- Product Development: Knowing the saponification value aids in developing new food products and improving existing ones, especially those involving fats like margarine and shortening.
Relevance in Cosmetics
In the cosmetics industry, both values are integral to formulating products such as creams, lotions, and other skincare items. The acid value helps ensure the stability and safety of the oils used, which is crucial for products applied to the skin. A low acid value is often a sign of purity and mildness, desirable traits in skincare formulations.
The saponification value is significant when developing soaps and other cleansers. It indicates the amount of lye (sodium hydroxide) needed to convert fats and oils into soap, ensuring the end product is effective yet gentle enough for skin contact.
- Product Safety: Ensures that the oils used are not too acidic, which could irritate the skin.
- Efficiency in Production: Provides precise measurements for ingredient ratios in soap making, optimizing the saponification process.
Case Studies
Example in Biodiesel Production
One of the most compelling applications of the saponification value is in the biodiesel industry. Biodiesel production involves the conversion of oils into methyl esters (biodiesel) and glycerol, a process heavily dependent on the saponification value. This value indicates the potential yield of biodiesel from different types of fats and oils.
- Feedstock Selection: Producers choose oils with higher saponification values because they typically yield more biodiesel per unit of oil.
- Process Optimization: Adjusting the saponification process can maximize output and efficiency, crucial in commercial biodiesel operations.
A specific case study involves a biodiesel plant that optimized its production by selecting feedstocks with higher saponification values. This adjustment allowed for a more efficient conversion rate and better use of resources, ultimately enhancing profitability and reducing waste.
Example in Soap Manufacturing
In soap manufacturing, the saponification value is directly linked to the quality and characteristics of the final product. A higher saponification value usually means that less lye is required to achieve complete saponification, which can lead to a milder soap. This is particularly important in the production of soaps intended for sensitive skin or specialized applications.
- Product Quality: High saponification values indicate that oils can efficiently transform into soap, leading to a smoother, more consistent product.
- Cost Management: Efficient saponification helps reduce the amount of lye needed, lowering production costs.
A notable case study involves a soap manufacturer that used oils with calculated saponification values to create a line of luxury soaps. By selecting oils with the right saponification values, the company was able to produce soaps that offered exceptional moisturizing properties, appealing to consumers looking for premium skincare products.
Frequently Asked Questions
What is Acid Value?
The acid value is a measure indicating the amount of free fatty acids present in fats and oils. It reflects the degradation or rancidity of the product, which is critical for quality assessments in edible oils and other fat-containing products.
How is Saponification Value Calculated?
The saponification value is calculated by determining the amount of base, typically potassium hydroxide, required to saponify a fixed quantity of fat or oil. This value is crucial for industries like soap making where it indicates the potential yield of soap from the fat used.
Why are These Values Important in Industries?
Acid and saponification values are indispensable for quality control in industries that process oils and fats. They help determine the freshness, purity, and suitability of raw materials for production, ensuring that the final products meet required quality standards.
Can Acid and Saponification Values Affect Product Shelf Life?
Yes, both values can significantly affect product shelf life. A high acid value often indicates that an oil is degrading, which can shorten its shelf life, while the saponification value can suggest how well a fat or oil will react in long-term storage conditions, particularly in non-edible products.
Conclusion
Understanding the acid and saponification values of oils and fats is more than a chemical assessment—it’s a crucial part of ensuring product quality and suitability across various industries. These metrics not only guide manufacturers in choosing the right materials but also in maintaining the standards necessary for consumer safety and satisfaction.
By keeping a close watch on these values, industry professionals can better manage their products’ lifecycle from production to shelf, ensuring that they deliver both quality and reliability. This attention to detail is what ultimately sustains consumer trust and builds the reputation of products in competitive markets.