Organic chemistry stands as a foundational pillar in the scientific community, guiding the synthesis and understanding of molecules that are vital for various applications, from pharmaceuticals to material science. Among the numerous compounds studied within this discipline, esters hold a particular significance due to their versatile applications. Specifically, Acetylacetoacetic Ester and Malonic Ester are two compounds that have garnered attention for their roles in synthetic organic chemistry.
The difference between Acetylacetoacetic Ester and Malonic Ester lies primarily in their chemical structures and the subsequent properties and applications that arise from these differences. Acetylacetoacetic Ester features an acetoacetic acid ester functional group, which is known for its ketone and ester functionalities. In contrast, Malonic Ester contains a malonic acid ester group, characterized by its two ester functions adjacent to a single methylene group. These structural variations significantly influence their reactivity, stability, and usability in synthesis reactions.
Both esters serve as crucial components in the synthesis of a wide array of organic compounds, offering pathways to complex molecular structures through different reaction mechanisms. Acetylacetoacetic Ester and Malonic Ester are instrumental in creating pharmaceuticals, dyes, and polymers, showcasing their indispensable role in advancing chemical research and industrial applications. Their unique properties enable chemists to explore innovative solutions to synthetic challenges, underlining the importance of understanding these compounds’ differences and similarities.

Core Chemistry
Acetylacetoacetic Ester Explained
Chemical Structure and Formula
Acetylacetoacetic Ester, also known as Acetoacetic Ester, is an organic compound with the chemical formula C6H10O3. Its structure comprises a ketone and an ester group, making it a versatile molecule in organic synthesis. The ketone group is located between two carbon atoms, while the ester group is attached to the carbon at the end of the molecule. This dual functionality is key to its reactivity and usefulness in synthesis.
Physical Properties
Acetylacetoacetic Ester is a colorless liquid at room temperature, with a distinctive fruity odor. It is soluble in most organic solvents, such as ethanol, methanol, and diethyl ether, but less soluble in water. This solubility profile is crucial for its application in organic synthesis, as it determines the solvent systems in which it can be used effectively.
Synthesis Methods
The synthesis of Acetylacetoacetic Ester typically involves the Claisen condensation reaction. The steps include:
- Combining ethyl acetate (as both the ester and the solvent) with sodium ethoxide, which acts as a strong base.
- This reaction forms sodium acetoacetate, which upon acidification yields Acetylacetoacetic Ester.
This method demonstrates the practical approach to synthesizing this compound, leveraging its inherent reactivity for the formation of more complex molecules.
Malonic Ester Explained
Chemical Structure and Formula
Malonic Ester, known formally as Diethyl Malonate, bears the chemical formula C7H12O4. It consists of two ester groups attached to a central methylene group. This structure is pivotal for its role in organic reactions, particularly in the synthesis of carboxylic acids and their derivatives through the Malonic Ester synthesis process.
Physical Properties
As a colorless liquid, Malonic Ester shares some physical properties with Acetylacetoacetic Ester, such as a pleasant odor and solubility in organic solvents. However, it has a higher boiling point, which reflects its slightly larger molecular size and the presence of two ester groups. These physical properties affect its handling and the conditions under which it can be reacted in synthetic applications.
Synthesis Methods
Malonic Ester is commonly synthesized through the esterification of malonic acid. The general steps include:
- Reacting malonic acid with an alcohol (usually ethanol) in the presence of an acid catalyst.
- The reaction yields Diethyl Malonate, with water as a byproduct.
This process highlights the flexibility of esters to be formed from their corresponding acids, a fundamental principle in organic chemistry.
Key Differences
Structural Variances
The primary difference between Acetylacetoacetic Ester and Malonic Ester lies in their molecular structures. Acetylacetoacetic Ester contains a ketone group in addition to an ester function, whereas Malonic Ester features two ester groups connected by a methylene chain. These structural differences are critical, as they directly influence the compounds’ reactivity, stability, and functional applications in organic synthesis.
Comparison of Molecular Structures
The ketone group in Acetylacetoacetic Ester enables enolization – a key reaction that contributes to its versatility in synthesis. On the other hand, the dual ester groups in Malonic Ester facilitate decarboxylation reactions, allowing for the creation of carboxylic acids with varied side chains.
Functional Groups and Their Implications
The presence of different functional groups in these esters dictates their reactivity patterns. Acetylacetoacetic Ester’s ketone group makes it more prone to nucleophilic attacks, while Malonic Ester’s ester groups make it suitable for alkylation followed by decarboxylation. Understanding these reactivity trends is vital for chemists designing synthesis pathways.
Chemical Properties
Acidity Levels
Both esters exhibit moderate acidity, attributed to the hydrogen atoms adjacent to the carbonyl (C=O) group. However, the acidity level of Malonic Ester is slightly higher due to the inductive effect of the two ester groups, which stabilize the carbanion formed upon deprotonation.
Stability and Reactivity
The stability of Acetylacetoacetic Ester and Malonic Ester varies under different conditions. Acetylacetoacetic Ester is less stable towards hydrolysis compared to Malonic Ester due to the ketone group’s presence. Reactivity wise, both esters are highly reactive in organic synthesis, offering pathways to diverse molecular structures.
Synthesis and Uses
Differences in Synthesis Processes
The synthesis of Acetylacetoacetic Ester and Malonic Ester involves distinct processes that highlight their unique chemical behaviors. Claisen condensation is crucial for Acetylacetoacetic Ester, while esterification of malonic acid is key to producing Malonic Ester. These methods reflect the compounds’ structural requirements and desired outcomes in synthesis.
Common Applications in Organic Synthesis
Acetylacetoacetic Ester and Malonic Ester are foundational in synthesizing carbonyl compounds, carboxylic acids, and their derivatives. They serve as building blocks in creating complex molecules, showcasing the breadth of their applications in organic chemistry.
Role in Pharmaceuticals and Material Science
Both esters are indispensable in the pharmaceutical industry, aiding in the synthesis of active pharmaceutical ingredients (APIs) and intermediates. Their role extends to material science, where they contribute to developing polymers and coatings, illustrating their versatility beyond organic synthesis.

Mechanisms of Reaction
Acetylacetoacetic Ester Reactions
Enolization and its Importance
Acetylacetoacetic Ester undergoes enolization, a crucial reaction where the keto form of the ester converts into its enol form. This equilibrium between keto and enol forms is pivotal for the compound’s reactivity, especially in condensation reactions. The enol form is more reactive towards electrophiles, enabling the formation of carbon-carbon bonds. Enolization is not just a reaction; it’s a gateway that opens up Acetylacetoacetic Ester to a broader spectrum of chemical transformations, crucial for synthesizing complex organic compounds.
Reaction with Nucleophiles
The keto form of Acetylacetoacetic Ester is particularly reactive towards nucleophiles due to the presence of a carbonyl group. This reactivity allows for the addition of nucleophiles, followed by subsequent reactions that can lead to the formation of a wide range of products. For instance, the treatment with sodium hydride can result in the formation of an enolate, which can then react with various electrophiles, creating a multitude of potential synthesis pathways.
Malonic Ester Reactions
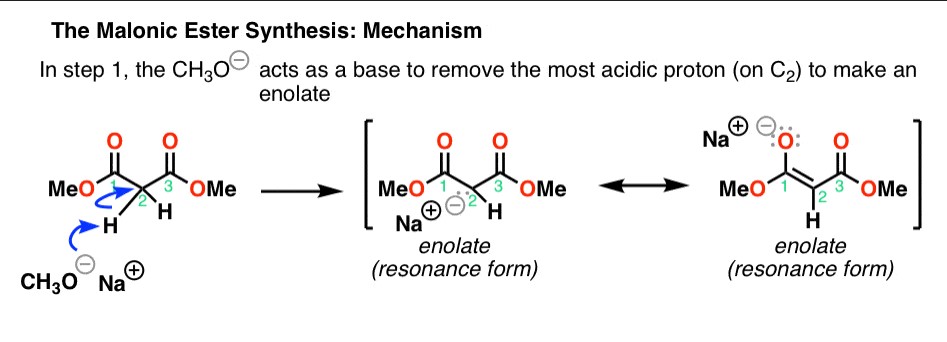
Decarboxylation Steps
Malonic Ester’s hallmark reaction is decarboxylation, a process where it loses a carbon dioxide molecule. This reaction is essential in synthesizing carboxylic acids and their derivatives. The decarboxylation of Malonic Ester typically involves:
- Activation of the ester with a base to form the carbanion.
- Alkylation of the activated ester.
- Heating the alkylated ester to induce decarboxylation, leading to the formation of a substituted acetic acid.
This stepwise transformation showcases the versatility of Malonic Ester in organic synthesis, providing a straightforward route to a variety of complex structures.
Reaction with Electrophiles
The activated form of Malonic Ester, being a strong nucleophile, readily reacts with electrophiles. This reactivity is exploited in alkylation reactions, where carbon chains or functional groups are introduced, leading to highly substituted carboxylic acids. These reactions are instrumental in building complex molecules, demonstrating Malonic Ester’s value in organic synthesis.
Comparative Analysis
In Organic Synthesis
How Each Contributes to Building Complex Molecules
Acetylacetoacetic Ester and Malonic Ester are cornerstone reagents in organic synthesis. Their unique reactivities—Acetylacetoacetic Ester’s enolization and Malonic Ester’s decarboxylation—offer distinct pathways for constructing complex molecules. These esters enable the introduction of varied functional groups and the formation of carbon-carbon bonds, crucial steps in synthesizing pharmaceuticals, agrochemicals, and polymers.
Examples of Synthesis Reactions
- Acetylacetoacetic Ester is used in the synthesis of pyridines and ketones, where its enol form reacts with various electrophiles.
- Malonic Ester serves as a precursor for the synthesis of barbiturates and carboxylic acids, utilizing its propensity for alkylation followed by decarboxylation.
In Industrial Applications
Use in Manufacturing and Product Development
Both esters find extensive applications in industrial settings. Acetylacetoacetic Ester is utilized in the manufacture of coatings and adhesives, benefiting from its reactivity to form stable bonds. Malonic Ester, on the other hand, is key in producing flavors, fragrances, and pharmaceuticals, where its ability to form diverse carboxylic acids is highly valued.
Economic Implications of Each Ester
The production and use of these esters have significant economic implications. The versatility and reactivity of Acetylacetoacetic and Malonic Esters reduce the number of steps required in synthesis processes, leading to cost savings and efficiency improvements in manufacturing. Their role in developing high-value products further underscores their economic importance.
Advantages and Limitations
Acetylacetoacetic Ester
Benefits in Synthetic Applications
Acetylacetoacetic Ester is highly valued for its dual functionality and the ability to undergo enolization, making it indispensable in synthetic applications. It enables the synthesis of ketones and carbonyl compounds, which are fundamental structures in many organic molecules.
Drawbacks and Challenges
However, its instability in certain conditions and susceptibility to hydrolysis can limit its utility. Additionally, the control of enolization and subsequent reactions requires precise conditions, which can complicate synthesis protocols.
Malonic Ester
Advantages in Compound Development
Malonic Ester’s ability to undergo smooth decarboxylation and alkylation makes it a powerful tool in the development of complex compounds, especially in the synthesis of carboxylic acids with diverse structures.
Limitations in Use
One limitation is the need for strict control over reaction conditions to prevent unwanted side reactions. Moreover, the sensitivity of Malonic Ester to hydrolysis can pose challenges in handling and storage.
Future Perspectives
Emerging Research Areas
Recent studies focus on catalysis and green chemistry approaches to enhance the reactivity and application scope of Acetylacetoacetic and Malonic Esters. Research into novel catalysts that can facilitate their reactions under milder conditions is gaining momentum, aiming to increase efficiency and reduce environmental impact.
Potential for New Applications and Technologies
The versatility of these esters suggests their potential in emerging technologies, such as biodegradable polymers and sustainable materials. Advances in chemical research may unlock new methodologies leveraging these esters, contributing to the development of innovative products and processes that align with sustainability goals.
Frequently Asked Questions
What is Acetylacetoacetic Ester?
Acetylacetoacetic Ester is an organic compound known for its dual functional groups: a ketone and an ester. This ester serves as a pivotal reagent in organic synthesis, facilitating the construction of complex molecules through its ability to undergo enolization and react with nucleophiles. Its versatility in reactions makes it an invaluable tool in the creation of pharmaceuticals and other organic compounds.
How is Malonic Ester used in synthesis?
Malonic Ester is utilized in organic synthesis for its unique ability to undergo decarboxylation, providing access to substituted acetic acids. This property is particularly beneficial for synthesizing carboxylic acids with various side chains, making Malonic Ester a key reagent in the production of pharmaceuticals, dyes, and perfumes. Its functionality allows for the straightforward introduction of complexity into molecules.
Why are esters important in organic chemistry?
Esters are crucial in organic chemistry due to their reactivity and versatility. They play a central role in numerous synthesis reactions, acting as intermediates in the creation of a wide range of organic molecules. Esters are fundamental in developing pharmaceuticals, plastics, and fragrances, highlighting their widespread applications across different industries. Their ability to participate in diverse chemical reactions makes them indispensable in organic synthesis.
Conclusion
Understanding the nuances between Acetylacetoacetic Ester and Malonic Ester reveals the intricate beauty of organic chemistry and its practical implications in real-world applications. These compounds exemplify how slight structural differences can lead to diverse pathways in synthesis, offering chemists the tools to craft complex molecules with precision and creativity. Their study not only enriches the academic field but also drives innovation in industries reliant on synthetic organic chemistry.
As we continue to explore the depths of chemical compounds and their interactions, the knowledge gained from examining Acetylacetoacetic Ester and Malonic Ester serves as a testament to the endless possibilities within organic synthesis. This exploration paves the way for future advancements in medicine, material science, and beyond, showcasing the transformative power of chemistry in shaping our world.

