Organic chemistry, the branch of science devoted to the study and manipulation of carbon-containing compounds, plays a pivotal role in the development of various industrial and pharmaceutical products. Central to this field are two key intermediates: carbocations and carbanions. These species are highly reactive and serve as fundamental building blocks in numerous chemical reactions, paving the way for the synthesis of complex molecules.
Carbocations are positively charged ions where a carbon atom holds an incomplete octet, making them highly electrophilic. Carbanions, in contrast, bear a negative charge on a carbon atom, resulting in a nucleophilic character. The behavior and stability of these ions are influenced by their surrounding environment, including the presence of other atoms and the electronic configuration of the molecule itself.
Understanding the properties and reactivities of carbocations and carbanions is crucial for chemists to manipulate and create new compounds. Their formation, stability, and participation in chemical reactions underpin many synthetic pathways, offering insights into the mechanisms that drive organic synthesis. This knowledge not only aids in the creation of novel substances but also in the refinement of existing synthetic methods, highlighting the significance of these intermediates in advancing the field of chemistry.
Carbocation Basics
Definition and Structure
A carbocation is an organic molecule where a carbon atom holds a positive charge. This scenario arises due to the carbon atom lacking sufficient electrons to form a stable set of bonds. Structurally, carbocations are sp2 hybridized, meaning the carbon atom is connected to three other atoms and has an empty p orbital, resulting in a planar shape.
Formation Processes
Carbocations primarily form through two processes:
- Ionization of alkyl halides: When alkyl halides react with a strong base, the halide ion leaves, forming a carbocation.
- Hydride shifts in reactions: During chemical reactions, a hydrogen atom might shift from one carbon to another, creating a positively charged carbon atom.
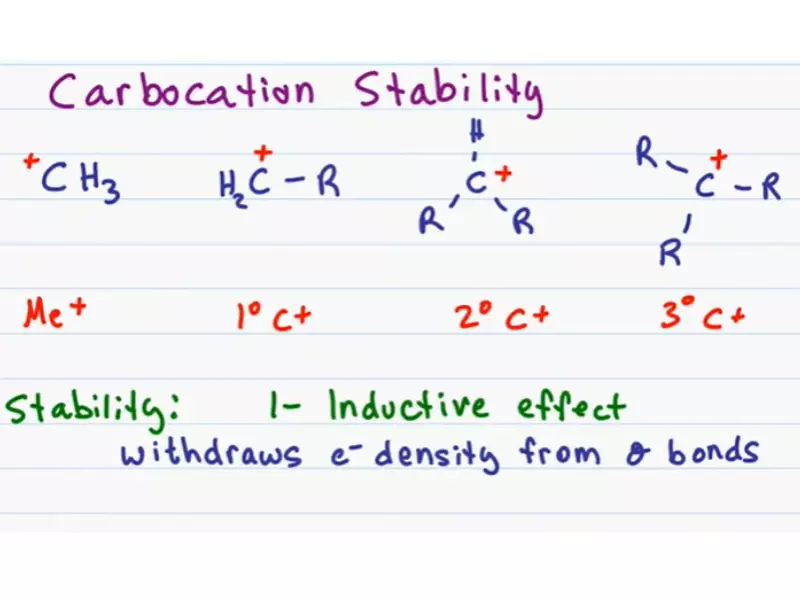
Types of Carbocations
Primary, Secondary, Tertiary
- Primary carbocation: Attached to one alkyl group.
- Secondary carbocation: Attached to two alkyl groups.
- Tertiary carbocation: Attached to three alkyl groups.
Allylic and Benzylic
- Allylic carbocations: Occur when the positive charge is adjacent to a double bond.
- Benzylic carbocations: Formed when the positive charge is on a carbon atom directly connected to a benzene ring.
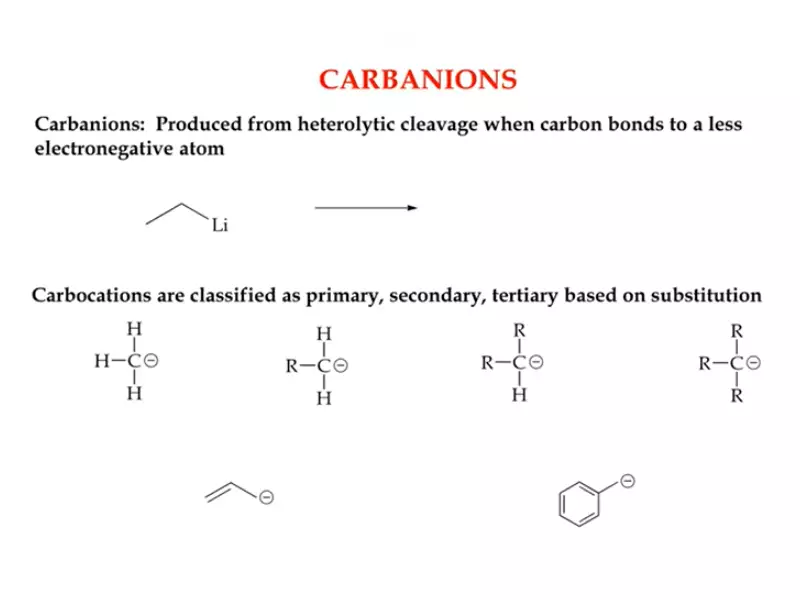
Carbanion Basics
Definition and Structure
A carbanion is the opposite of a carbocation; it is an organic molecule where a carbon atom has a negative charge. This occurs when the carbon atom has an extra pair of electrons. Structurally, carbanions can be sp3 hybridized, indicating a tetrahedral geometry, although variations exist based on the nature of the substituents.
Formation Processes
Carbanions form through:
- Deprotonation of organic molecules: Removal of a hydrogen atom as a proton, leaving a pair of electrons behind.
- Organometallic reactions: Reaction of organic compounds with metals can result in a negatively charged carbon atom.
Types of Carbanions
Methyl, Primary, Secondary, Tertiary
- Methyl carbanion: A single carbon atom with three hydrogens and a negative charge.
- Primary carbanion: Attached to one alkyl group with a negative charge.
- Secondary carbanion: Attached to two alkyl groups with a negative charge.
- Tertiary carbanion: Attached to three alkyl groups, rarely stable due to steric hindrance.
Allylic and Benzylic
- Allylic carbanions: Occur next to a double bond, stabilized by resonance.
- Benzylic carbanions: Occur when the negatively charged carbon is directly connected to a benzene ring, also stabilized by resonance.
Stability Factors
Carbocation Stability
- Inductive effect: Electron-donating groups increase stability by reducing the charge.
- Resonance stabilization: Delocalization of the positive charge over a molecule increases stability.
- Hyperconjugation: Sharing of electrons from neighboring C-H bonds can stabilize the carbocation.
- Hybridization and orbital structure: Carbocations are more stable if the positive charge resides in an orbital closer to s-character.
Carbanion Stability
- Inductive effect: Electron-withdrawing groups increase stability by delocalizing the charge.
- Resonance stabilization: Negative charge spread over multiple atoms increases stability.
- Hybridization and orbital structure: sp3 hybridized carbanions are more stable due to the increased s-character of the orbital holding the lone pair.
Reaction Mechanisms
Carbocation Reactions
Addition Reactions
Carbocations often participate in addition reactions where they react with nucleophiles to form a more stable product.
Substitution Reactions
Carbocations can undergo substitution reactions, where they exchange one group for another, often in the presence of a nucleophile.
Rearrangements
Rearrangements occur when a carbocation undergoes structural changes to form a more stable carbocation.
Carbanion Reactions
Nucleophilic Addition
Carbanions, being rich in electrons, readily add to electron-deficient carbon atoms in a process known as nucleophilic addition.
Nucleophilic Substitution
In nucleophilic substitution, carbanions replace a leaving group in organic molecules, forming new bonds.
Elimination Reactions
Elimination reactions involve carbanions removing atoms from adjacent carbon atoms, often leading to the formation of double or triple bonds.
Comparative Analysis
Reactivity Patterns
Carbocations and carbanions exhibit distinct reactivity patterns due to their opposite charges. Carbocations, with a positive charge, are electron-deficient, making them highly reactive towards nucleophiles or electron-rich species. This characteristic underlies their prominent role in electrophilic addition and substitution reactions. Conversely, carbanions, bearing a negative charge, are electron-rich and thus seek electron-deficient sites, making them strong nucleophiles. They predominantly partake in nucleophilic addition and substitution reactions, displaying a fundamental difference in behavior compared to carbocations.
Role in Organic Synthesis
The role of carbocations and carbanions in organic synthesis cannot be overstated. Carbocations are pivotal in forming new carbon-carbon (C-C) bonds, essential for constructing complex molecules. For instance, they are key intermediates in the Friedel-Crafts alkylation and acylation reactions, crucial for synthesizing aromatic compounds. On the other hand, carbanions enable the formation of carbon-heteroatom bonds, as seen in the synthesis of ketones and aldehydes through nucleophilic addition to carbonyl groups. Their behavior dictates the strategies chemists employ in synthesizing a vast array of organic compounds.
Influence of Solvents
The influence of solvents on the stability and reactivity of carbocations and carbanions is significant. Solvents can stabilize or destabilize these intermediates, affecting the overall reaction outcome. Polar protic solvents, for instance, can stabilize carbocations through solvation, enhancing their formation and reactivity. In contrast, polar aprotic solvents are better at stabilizing carbanions, as they can solvate cations without donating hydrogen bonds to the negatively charged intermediates, thereby increasing their reactivity in nucleophilic reactions.
Practical Applications
Pharmaceutical Synthesis
The synthesis of pharmaceuticals heavily relies on reactions involving carbocations and carbanions. For example, carbocations play a crucial role in the synthesis of steroidal drugs through cyclization reactions, where multiple ring systems are formed. Carbanions contribute to the creation of many life-saving drugs, including antiviral medications, through the formation of C-C bonds in key steps of their synthesis. The ability to manipulate these intermediates with precision enables the development of complex molecules with therapeutic benefits.
Polymer Industry
In the polymer industry, both carbocations and carbanions serve as initiators in polymerization reactions. Carbocationic polymerization is a method used to synthesize polymers like polyisobutylene, where carbocations act as the propagating species. Similarly, anionic polymerization, involving carbanions, is employed to create polymers with specific structures and properties, such as styrene-butadiene rubber (SBR), crucial for manufacturing tires. The control over molecular weight and the polymer structure is paramount in developing materials with desired characteristics.
Agrochemicals
The development of agrochemicals, such as pesticides and herbicides, often involves carbocation and carbanion chemistry. Carbocations are utilized in the synthesis of certain herbicides, where the selective formation of key intermediates allows for the effective targeting of weed species. Carbanions contribute to the design of novel insecticides by enabling the construction of complex molecules that can interact with biological targets in pests, disrupting their life cycle and preventing crop damage.
Challenges and Solutions
Managing Reactivity and Selectivity
One of the primary challenges in working with carbocations and carbanions lies in their high reactivity, which can lead to side reactions and decreased selectivity. To manage this, chemists employ various strategies:
- Use of specific solvents that can enhance or suppress reactivity.
- Temperature control to slow down fast-reacting intermediates.
- Design of reaction pathways that favor the desired outcome through the use of protective groups or directing groups.
Advanced Synthesis Techniques
Advanced synthesis techniques have been developed to harness the potential of carbocations and carbanions in a controlled manner. These include:
- Flow chemistry, which allows for precise control over reaction conditions, improving yield and selectivity.
- Catalysis, both metal-based and organocatalysis, to enhance reaction rates and selectivity.
- Computational chemistry tools to predict reactivity patterns and optimize reaction conditions.
Future Perspectives
Innovative Approaches in Synthesis
Looking ahead, innovative approaches in the synthesis involving carbocations and carbanions are poised to revolutionize organic chemistry. Techniques like photoredox catalysis offer new pathways to generate these intermediates under mild conditions, expanding the toolbox for molecular construction.
Computational Chemistry Advancements
Advancements in computational chemistry will further our understanding of the intricate dynamics of carbocation and carbanion chemistry. Predictive models and simulations are becoming increasingly accurate, enabling the design of reactions with unprecedented precision and efficiency.
Environmental Impact and Sustainability
Lastly, the environmental impact and sustainability of chemical synthesis involving carbocations and carbanions are gaining attention. Green chemistry principles are being applied to minimize waste and reduce the use of hazardous reagents, aiming for a future where organic synthesis is both innovative and environmentally responsible.
Frequently Asked Questions
What is a Carbocation?
A carbocation is a positively charged ion with a carbon atom that has only six electrons in its outer shell, creating an electrophilic site that seeks to gain electrons. This property makes carbocations highly reactive, playing a critical role in various chemical reactions, including organic synthesis and rearrangement processes.
What is a Carbanion?
A carbanion is an anionic species featuring a carbon atom bearing a negative charge, resulting from the presence of a lone pair of electrons. This configuration imparts a nucleophilic nature to the carbanion, enabling it to donate electrons in chemical reactions. Carbanions are essential in numerous synthetic pathways, such as nucleophilic substitutions and base-catalyzed eliminations.
How does the stability of carbocations and carbanions vary?
The stability of carbocations and carbanions depends on several factors, including the inductive effect, resonance stabilization, hyperconjugation, and the hybridization state of the carbon atom. Carbocations are stabilized by electron-donating groups and delocalization of charge, while carbanions are stabilized through electron-withdrawing groups and resonance.
Why are carbocations and carbanions important in organic synthesis?
Carbocations and carbanions are pivotal in organic synthesis due to their high reactivity, which allows them to participate in a wide range of chemical reactions. They are involved in key processes such as forming new carbon-carbon bonds, rearranging molecular structures, and facilitating functional group transformations, making them indispensable tools in the chemist’s toolkit.
Conclusion
Carbocations and carbanions embody the reactive heart of organic chemistry, facilitating a myriad of transformations that are foundational to synthesizing the complex molecules that underpin modern society. Their study not only enables the creation of new drugs and materials but also deepens our understanding of chemical reactivity and mechanism.
The exploration of these charged intermediates continues to reveal new pathways and techniques, driving innovation in synthesis and beyond. As we refine our understanding and control over these entities, the boundaries of what can be achieved through organic chemistry continue to expand, promising exciting developments in various fields of science and industry.
