Electrolysis represents a fundamental technique in the realm of chemical manufacturing and energy solutions, pivotal for breaking down substances into their core components using electric current. This process harnesses the power of electricity to initiate chemical reactions that would not otherwise occur spontaneously. The applications of electrolysis span from large-scale industrial productions to critical scientific research, underscoring its versatility and importance.
Molten and aqueous electrolysis are two primary methods, each serving distinct purposes and utilizing different setups. Molten electrolysis involves the decomposition of a pure molten compound, generally at high temperatures, to release pure elements. Conversely, aqueous electrolysis occurs in a solution where water participates actively in the electrochemical reactions, often leading to the production of hydrogen and oxygen gases.
While both techniques achieve similar end goals— the separation of elements— their operational conditions, efficiency, and application areas differ significantly. Molten electrolysis typically operates at higher temperatures and is ideal for extracting metals from raw ores. Aqueous electrolysis, on the other hand, is crucial for sustainable energy solutions like hydrogen production.
Electrolysis Basics
What is Electrolysis?
Electrolysis is a vital chemical process where an electric current drives a chemical reaction that would not occur under normal circumstances. This technique is pivotal for separating elements and molecules from their compounds, which plays a crucial role in various industrial and scientific applications.
Components of an Electrolytic Cell
An electrolytic cell, fundamental to the process of electrolysis, includes several essential components:
- Electrodes: Two electrodes, an anode (positive) and a cathode (negative), are involved. The anode is where oxidation (loss of electrons) occurs, while reduction (gain of electrons) happens at the cathode.
- Electrolyte: This is the medium that carries ions in the cell and can be either a molten salt or an aqueous solution. The electrolyte must conduct electricity, allowing ions to move between electrodes.
- Power Source: A battery or another DC power source is connected to the electrodes to provide the energy necessary for the electrolysis to occur.
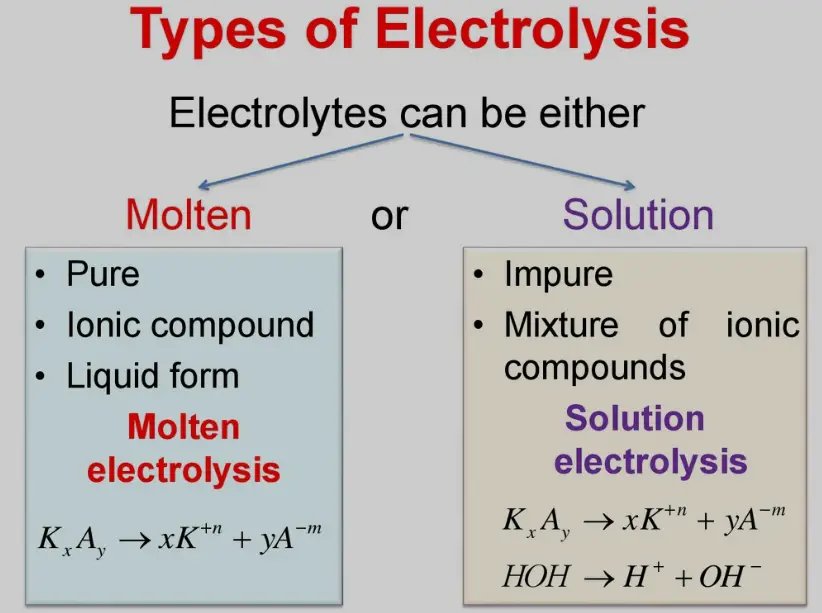
Types of Electrolysis
Electrolysis is categorized mainly based on the type of electrolyte used in the process:
- Molten Electrolysis: Uses a molten ionic compound as the electrolyte.
- Aqueous Electrolysis: Involves an electrolyte that is a solution, where water is the solvent.
Comparison Table:
| Type | Electrolyte State | Common Uses | Temperature Requirements |
|---|---|---|---|
| Molten Electrolysis | Molten (liquid state of ionic compounds) | Extraction of metals like aluminum and sodium | High, enough to melt the compound |
| Aqueous Electrolysis | Solution (water as solvent) | Production of gases like hydrogen and oxygen | Typically lower, close to room temperature |
Molten Electrolysis
Process Description
Molten electrolysis involves the decomposition of a pure molten compound to extract its elements. This method is particularly effective for metals that are too reactive to be extracted using more traditional means.
Setup and Conditions
Setting up a molten electrolysis involves:
- Heating: The electrolyte needs to be heated to a high temperature until it melts.
- Electrodes: Typically made from inert materials to withstand the high temperatures and corrosive nature of the molten salts.
Electrolytes Used
Common electrolytes in molten electrolysis include:
- Sodium chloride (NaCl)
- Calcium chloride (CaCl2) These salts are chosen because they can conduct electricity effectively when molten.
Applications
Molten electrolysis is used in:
- Metal Extraction: Metals like aluminum and sodium are commercially produced using this method.
- Metal Refining: Purifying metals such as titanium through the molten salt electrolysis method.
Advantages of Molten Electrolysis
- Energy Efficiency: Typically more energy-efficient for metals that require high energy inputs using other methods.
- Purity of Product: Produces very high-purity metals, essential for specific industrial applications.
Aqueous Electrolysis
Process Overview
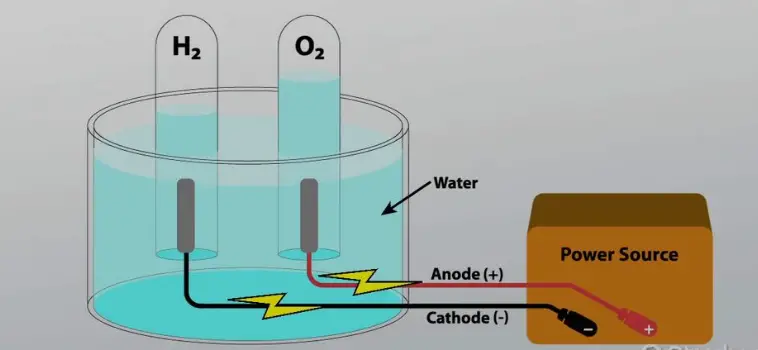
Aqueous electrolysis involves the electrolysis of water or solutions containing water, where water itself is one of the reactants in the electrolysis.
Setup and Conditions
A typical setup for aqueous electrolysis includes:
- Standard Pressure and Temperature: Operates effectively at atmospheric pressure and moderate temperatures.
- Inert Electrodes: Such as platinum or stainless steel, which do not react with the electrolyte.
Common Electrolytes
Electrolytes commonly used in aqueous electrolysis include:
- Sulfuric acid (H2SO4)
- Sodium hydroxide (NaOH) These are selected for their ability to dissociate completely into ions.
Applications
Aqueous electrolysis is critical for:
- Hydrogen Production: Mainly used in generating hydrogen for fuel cells.
- Environmental Clean-up: Used in treating waste water by breaking down pollutants.
Role in Water Splitting
Water splitting in aqueous electrolysis typically produces hydrogen at the cathode and oxygen at the anode, a process critical for sustainable energy solutions.
Benefits in Environmental Technology
- Sustainable Energy: Produces hydrogen, which is a clean and renewable energy source.
- Pollution Reduction: Helps reduce environmental pollutants through the decomposition of harmful substances in water.
Key Differences
Temperature and Setup
Comparison of Operational Temperatures
Molten and aqueous electrolysis processes differ significantly in their operational temperatures. Molten electrolysis requires high temperatures to melt the electrolyte, often exceeding 800°C, depending on the melting point of the salt used. In contrast, aqueous electrolysis typically operates at much milder temperatures, usually around room temperature to slightly above, making it more suitable for a broader range of environments.
Equipment Differences
The equipment used in each type of electrolysis also varies due to these temperature differences:
- Molten Electrolysis: Requires heat-resistant materials and robust insulation to maintain high temperatures.
- Aqueous Electrolysis: Utilizes more standard laboratory or industrial equipment, with less emphasis on heat tolerance and more on corrosion resistance due to the aqueous environment.
Electrolytes and Reactions
Types of Ions Involved
The ions involved in each type of electrolysis differ based on the electrolyte:
- Molten Electrolysis: Primarily involves simple ions from the molten salts, such as Na+ or Cl-.
- Aqueous Electrolysis: Deals with a variety of ions, including H+ and OH- from water, along with any ions from dissolved salts or acids.
Reaction Products for Each Method
The products of the reactions also vary:
- Molten Electrolysis: Typically results in the production of elemental metals and nonmetals like oxygen or chlorine.
- Aqueous Electrolysis: Commonly produces hydrogen and oxygen gases from the electrolytic splitting of water.
Efficiency and Cost
Energy Consumption Comparison
Energy consumption in molten electrolysis is generally higher due to the heat required to melt the electrolyte. However, this is offset by the typically higher efficiency of the process in terms of yield and purity of the extracted metals.
Cost-Effectiveness in Long Term
Cost-effectiveness of each method depends on the scale and purpose of the electrolysis:
- Molten Electrolysis: More cost-effective for large-scale metal production due to the high throughput and lower relative energy costs at scale.
- Aqueous Electrolysis: More advantageous for smaller-scale operations, especially where environmental impact and energy consumption are concerns.
Advantages and Limitations
Benefits of Each Method
Specific Advantages in Various Applications
- Molten Electrolysis: Ideal for the extraction and refinement of highly reactive metals, providing a method to obtain metals like aluminum and sodium with high purity and efficiency.
- Aqueous Electrolysis: Best suited for applications requiring the production of gases, such as hydrogen for fuel cells, due to its relatively simple setup and lower temperature operations.
Limitations and Challenges
Technical and Economic Barriers
- Molten Electrolysis: Faces challenges such as the high cost of initial setup and maintenance due to the aggressive and high-temperature environment.
- Aqueous Electrolysis: While less costly to set up, struggles with lower efficiencies and the need for frequent maintenance due to electrode degradation.
Future Perspectives
Technological Advances
Innovations in Electrolysis Technology
Recent technological advances are aimed at increasing the efficiency and reducing the operational costs of both types of electrolysis. Innovations include development of more durable and efficient electrode materials, as well as improved electrolyte formulations that can operate at lower temperatures and higher efficiencies.
Environmental Impact
Contribution to Sustainable Practices
Both methods of electrolysis contribute significantly to sustainable industrial practices:
- Molten Electrolysis: Reduces the need for traditional mining and refining, which can be environmentally damaging.
- Aqueous Electrolysis: Supports the shift to renewable energy sources by providing a method to produce hydrogen, a clean fuel, from water.
Frequently Asked Questions
What is electrolysis?
Electrolysis is a chemical process in which electric current is passed through a substance to cause a chemical change. This technique is especially useful for decomposing chemical compounds, often resulting in the liberation of gases or the deposition of elements.
How does molten electrolysis differ from aqueous electrolysis?
Molten electrolysis involves passing an electric current through a molten ionic compound to decompose it into its elements, typically used for metal extraction. Aqueous electrolysis, however, uses water as the solvent and is primarily used for the production of hydrogen and oxygen.
What are the applications of aqueous electrolysis?
Aqueous electrolysis is widely used in the production of hydrogen gas, which is a clean fuel alternative. It also plays a crucial role in various industrial processes requiring pure hydrogen, such as in ammonia synthesis for fertilizer production and as a reducing agent in metals refining.
Why is molten electrolysis important in industry?
Molten electrolysis is critical for extracting metals from their natural ore forms, particularly for reactive metals like sodium and aluminum. This process provides a more energy-efficient and environmentally friendly alternative to more traditional methods involving carbon or other reducing agents.
Conclusion
Electrolysis, both molten and aqueous, serves as a cornerstone in the technological advancements of chemical processing and energy production. The choice between molten and aqueous electrolysis depends on the specific requirements of the process, including the desired products, efficiency, cost, and environmental impact. By understanding the fundamental differences and applications of each method, industries can optimize their processes and contribute to more sustainable practices.
Looking forward, the innovations in electrolysis technology are expected to enhance efficiency and reduce costs, further solidifying its role in modern industrial applications. As the world continues to seek greener and more sustainable technological solutions, the evolution of electrolysis will be paramount in meeting these environmental and economic challenges.